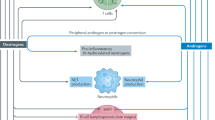
Abstract
Use of contraceptives by women with rheumatic diseases, especially those with systemic lupus erythematosus, has long been thought to carry risks, such as disease exacerbation, thrombosis and other adverse effects. The use of effective contraception has, therefore, been avoided, despite many affected women being of reproductive age. Knowledge of risks and benefits of contraceptive methods in the general population has improved, as have the safety and effectiveness of hormonal contraceptives. Methods of administration have evolved and now include transdermal and intravaginal routes, a progesterone-releasing intrauterine device, and an extended-cycle oral contraceptive. Birth control pills are not all alike; the risk of adverse effects varies depending on the amount of estrogen and type of progestin used. Data show that patients with stable systemic lupus erythematosus are not at increased risk of disease flare while taking standard oral contraceptives. Despite a lack of randomized studies, evidence strongly suggests that the elevated risk of thrombosis makes estrogen-containing contraceptives unsuitable for patients with antiphospholipid antibody. Other important issues include potential interactions between hormonal contraceptives and other medications and possible risk of infection if an intrauterine device is used. Rheumatologists are increasingly working with gynecologists and patients to make choices about which contraceptive methods to use. Decisions should be individualized according to the patient's medical status, personal preference, and stage of reproductive life.
Key Points
-
Most patients with rheumatic disease are able to choose a safe and effective contraceptive from the options available
-
Combined oral contraceptives do not increase risk of disease flare in patients with stable systemic lupus erythematosus
-
Estrogen-containing contraceptives are contraindicated in patients at increased risk for thrombosis, including patients who test positive for antiphospholipid antibody
-
Progesterone-only contraceptives—oral, intramuscular, or intrauterine device—do not increase risk of thrombosis risk and are recommended for patients who have a contraindication to estrogen
-
Medroxyprogesterone acetate and the Mirena® intrauterine device decrease menstrual blood flow in patients receiving warfarin
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Family planning, contraception, sterilization and pregnancy termination (2001) In: Comprehensive Gynecology, edn 4, 295–358 (Eds Stenchever MA et al.) St. Louis: Mosby, Inc.
Chez RA and Strathman I (1999) Contraception and Sterilization. In: Danforth's Obstetrics and Gynecology, 553–566 (Eds JR Scott et al.) Philadelphia: Lippincott, Williams and Wilkins
World Health Organization (2004) Medical eligibility criteria for contraceptive use (Third edition) [http://www.who.int/reproductive-health/publications/mec/index.htm] (accessed July 13, 2006)
Kaunitz AM (2005) Beyond the pill: new data and options in hormonal and intrauterine contraception. Am J Obstet Gynecol 192: 998–1004
The Practice Committee of the American Society for Reproductive Medicine (2004) Hormonal contraception: recent advances and controversies. Fertil Steril 82: 520–526
Herndon EJ and Zieman M (2004) New contraceptive options. Am Fam Physician 69: 853–860
U.S. Food and Drug Administration (2005) Safety alert: Ortho Evra (posted 11/14/2005) [http://www.fda.gov/medwatch/SAFETY/2005/safety05.htm] (accessed July 13, 2006)
Bigrigg A et al. (2000) Depo Provera. Discussion paper on clinical use, effectiveness and side effects. Br J Fam Plann 26: 97–99
Cundy T et al. (1994) Recovery of bone density in women who stop using medroxyprogesterone acetate. BMJ 308: 247–248
Burkman R et al. (2004) Safety concerns and health benefits associated with oral contraception. Am J Obstet Gynecol 190: S5–S22
Moreno V et al. (2002) Effect of oral contraceptives on risk of cervical cancer in women with human papilloma virus infection: the IARC multicentric case–control study. Lancet 359: 1085–1092
Collaborative Group on Hormonal Factors in Breast Cancer (1996) Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet 347: 1713–1727
Martinelli I (2001) Risk factors in venous thromboembolism. Thromb Haemost 86: 395–403
Gerstman BB et al. (1991) Oral contraceptive estrogen dose and the risk of venous thromboembolic disease. Am J Epidemiol 133: 32–37
Kemmeren JM et al. (2001) Third generation oral contraceptives and risk of venous thrombosis: meta-analysis. BMJ 323: 131–134
Rosing J et al. (2001) Oral contraceptives, thrombosis, and hemostasis. Eur J Obstet Gynecol Reprod Biol 95: 193–197
Lidegaard O et al. (2002) Oral contraceptives and venous thromboembolism: a five-year national case–control study. Contraception 65: 187–196
Voora D and Vijayan A (2003) Mesenteric vein thrombosis associated with intravaginal contraceptives: a case report and review of the literature. J Thromb Thrombolysis 15: 105–108
Abdollahi M et al. (2003) Obesity: risk of venous thrombosis and the interaction of coagulation factor levels and oral contraceptives. Thromb Haemost 89: 493–498
Kemmeren JM et al. (2002) Risk of Arterial Thrombosis in Relation to Oral Contraceptives (RATIO) study: oral contraceptives and the risk of ischemic stroke. Stroke 33: 1202–1208
Tanis BC et al. (2001) Oral contraceptives and the risk of myocardial infarction. N Engl J Med 345: 1787–1793
Lidegaard O and Kreiner S (2002) Contraceptives and cerebral thrombosis: a five-year national case–control study. Contraception 65: 197–205
Pettiti DB et al. (1996) Stroke in users of low dose contraceptives. N Engl J Med 335: 8–15
World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception (1997) Acute myocardial infarction and combined oral contraceptives: results of an international, multicentre, case–control study. Lancet 349: 1202–1209
Van den Bosch MA et al. (2003) The RATIO study: oral contraceptives and the risk of peripheral arterial disease in young women. J Thromb Haemost 1: 439–444
Bloemenkamp KWM et al. (2000) Higher risk of venous thrombosis during early use of oral contraceptives in women with inherited clotting defects. Arch Intern Med 160: 49–52
Forastiero R et al. (2001) The combination of thrombophilic genotypes is associated with definite antiphospholipid syndrome. Haematologica 86: 735–741
Brouwer JL et al. (2004) The contribution of inherited and acquired thrombophilic defects, alone or combined with antiphospholipid antibodies, to venous and arterial thromboembolism in patients with systemic lupus erythematosus. Blood 104: 143–148
Girolami A et al. (1996) Thromboembolic disease developing during oral contraceptive therapy in young females with antiphospholipid antibodies. Blood Coagul Fibrinolysis 7: 497–501
Petri M et al. (2005) Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med 353: 2550–2558
Sanchez-Guerrero J et al. (2005) A trial of contraceptive methods in women with systemic lupus erythematosus. N Engl J Med 353: 2539–2549
Schaedel ZE et al. (2005) The use of the levonorgestrel-releasing intrauterine system in the management of menorrhagia in women with hemostatic disorders. Am J Obstet Gynecol 193: 1361–1363
Lahita RG et al. (1979) Alterations of estrogen metabolism in systemic lupus erythematosus. Arthritis Rheum 22: 1195–1198
Garovich M et al. (1980) Oral contraceptives and systemic lupus. Arthritis Rheum 23: 1396–1398
Ansar Ahmend S et al. (1985) Sex hormones, immune responses, and autoimmune diseases. Mechanisms of sex hormone action. Am J Pathol 121: 531–551
Sanchez-Guerrero J et al. (1997) Past use of oral contraceptives and the risk of developing systemic lupus erythematosus. Arthritis Rheum 40: 804–808
Cooper GS et al. (2002) Hormonal and reproductive risk factors for development of systemic lupus erythematosus: results of a population based, case-control study. Arthritis Rheum 46: 1830–1839
Bengtsson AA et al. (2002) Risk factors for developing systemic lupus erythematosus: a case-control study in southern Sweden. Rheumatology (Oxford) 41: 563–571
Julkunen HA (1991) Oral contraceptives in systemic lupus erythematosus: side effects and influence on the activity of SLE. Scand J Rheumatol 20: 427–433
Jungers P et al. (1982) Influence of oral contraceptive therapy on the activity of systemic lupus erythematosus. Arthritis Rheum 25: 618–623
Mintz G et al. (1984) Contraception with progestagens in systemic lupus erythematosus. Contraception 30: 29–38
Buyon JP et al. (1995) Can women with systemic lupus erythematosus safely use exogenous estrogens? J Clin Rheumatol 1: 205–212
Ostensen M and Husby G (1983) A prospective study of the effect of pregnancy on rheumatoid arthritis and ankylosing spondylitis. Arthritis Rheum 26: 1155–1159
Spector TD and Hochberg MC (1990) The protective effect of the oral contraceptive pill on rheumatoid arthritis: an overview of the analytic studies using meta-analysis. J Clin Epidemol 43: 1221–1230
Pladevall-Vila M et al. (1996) Controversy of oral contraceptives and risk of rheumatoid arthritis: meta-analysis of conflicting studies and review of conflicting meta-analysis with special emphasis on analysis of heterogeneity. Am J Epidemiol 144: 1–14
Anonymous (1978) Reduction in incidence of rheumatoid arthritis associated with oral contraceptives. Royal College of General Practitioners' Oral Contraception Study. Lancet 1: 569–571
Spector TD et al. (1990) The pill, parity, and rheumatoid arthritis. Arthritis Rheum 33: 782–789
Jorgensen C et al. (1996) Oral contraception, parity, breast feeding, and severity of rheumatoid arthritis. Ann Rheum Dis 55: 94–98
Hernandez-Avila M et al. (1990) Exogenous sex hormones and the risk of rheumatoid arthritis. Arthritis Rheum 33: 947–953
Cutolo M et al. (2002) Androgens and estrogens modulate the immune and inflammatory responses in rheumatoid arthritis. Ann NY Acad Sci 966: 131–142
Booij A et al. (1996) Androgens as adjuvant treatment in postmenopausal patients with rheumatoid arthritis. Ann Rheum Dis 55: 811–815
Hall GM et al. (1994) A randomized controlled trial of the effect of hormone replacement therapy on disease activity in postmenopausal RA. Ann Rheum Dis 53: 112–116
Lekakis J et al. (1996) Acute estrogen administration can reverse cold-induced coronary Raynaud's phenomenon in systemic sclerosis. Clin Exp Rheumatol 14: 421–424
Bartelink ML et al. (1992) Raynaud's phenomenon: subjective influence of female sex hormones. Int Angiol 11: 309–315
Fotherby K (1990) Interactions with oral contraceptives. Am J Obstet Gynecol 163: 2153–2159
Trussell J (2004) Contraceptive Efficacy. In: Contraceptive Technology, edn 18 (Eds Hatcher RA et al.) New York: Ardent Media
Archer JSM and Archer DF (2002) Oral contraceptive efficacy and antibiotic interaction: a myth debunked. J Am Acad Dermatol 46: 917–923
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Sammaritano, L. Therapy Insight: guidelines for selection of contraception in women with rheumatic diseases. Nat Rev Rheumatol 3, 273–281 (2007). https://doi.org/10.1038/ncprheum0484
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncprheum0484
This article is cited by
-
Rheumatic Diseases in Reproductive Age—the Possibilities and the Risks
Reproductive Sciences (2023)
-
2021 recommendations of the Brazilian Society of Rheumatology for the gynecological and obstetric care of patients with Sjogren’s syndrome
Advances in Rheumatology (2021)
-
Managing pregnancy in inflammatory rheumatological diseases
Arthritis Research & Therapy (2011)
-
Contraception counseling in SLE—an often forgotten duty?
Nature Reviews Rheumatology (2011)