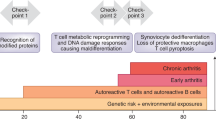
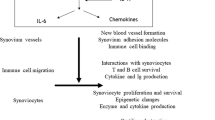
Key Points
-
Acute inflammation resolves actively through the engagement of specific proresolving mediators and mechanisms
-
The effect of failed resolution in chronic inflammatory settings is markedly unappreciated
-
Novel technologies afford analytical definition of proresolving mediators in clinical samples, providing new opportunities for stratification and target identification
-
Therapeutic strategies based on the biology of resolution can guide the development of novel medicines for the clinical management of inflammatory arthritis
-
Resolution-based therapies might be less burdened by unwanted effects than existing therapies and would activate tissue-protective mechanisms within patients
Abstract
The past two decades have witnessed major advancements in the clinical management of inflammatory arthritis, with new treatment strategies in some cases providing a marked improvement in patient outcomes. However, it is widely accepted that current strategies do not provide the 'total therapeutic solution', in view of the proportion of patients who do not respond to therapy, the important incidence of adverse effects and the development of an immune response against antibodies or fusion proteins used therapeutically. Moreover, although some therapeutic approaches can effectively bring about an end to inflammation, mechanisms to promote the recovery and/or repair of damage are required. Harnessing the concepts and mechanisms of the resolution of inflammation is a new approach to the treatment of inflammatory pathologies; this approach could help address the unmet need for new therapeutic approaches that not only control but also revert the course of inflammatory rheumatic diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Serhan, C. N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 (2014).
Dalli, J. et al. Identification and actions of a novel third maresin conjugate in tissue regeneration: MCTR3. PLoS ONE 11, e0149319 (2016).
Medzhitov, R. Origin and physiological roles of inflammation. Nature 454, 428–435 (2008).
Majno, G. Inflammation and infection: historic highlights. Monogr. Pathol. 1–17 (1982).
Nourshargh, S. & Alon, R. Leukocyte migration into inflamed tissues. Immunity 41, 694–707 (2014).
Perretti, M. Endogenous mediators that inhibit the leukocyte–endothelium interaction. Trends Pharmacol. Sci. 18, 418–425 (1997).
Willis, D., Moore, A. R., Frederick, R. & Willoughby, D. A. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat. Med. 2, 87–90 (1996).
Gilroy, D. W. et al. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 5, 698–701 (1999).
Liu, Y. et al. Glucocorticoids promote nonphlogistic phagocytosis of apoptotic leukocytes. J. Immunol. 162, 3639–3646 (1999).
Mitchell, S. et al. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J. Am. Soc. Nephrol. 13, 2497–2507 (2002).
El Kebir, D., Gjorstrup, P. & Filep, J. G. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc. Natl Acad. Sci. USA 109, 14983–14988 (2012).
Serhan, C. N., Chiang, N. & Dalli, J. The resolution code of acute inflammation: novel pro-resolving lipid mediators in resolution. Semin. Immunol. 27, 200–215 (2015).
Buckley, C. D., Gilroy, D. W. & Serhan, C. N. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 40, 315–327 (2014).
Ortega-Gomez, A., Perretti, M. & Soehnlein, O. Resolution of inflammation: an integrated view. EMBO Mol. Med. 5, 661–674 (2013).
Gilroy, D. & De Maeyer, R. New insights into the resolution of inflammation. Semin. Immunol. 27, 161–168 (2015).
Bertolotto, M. et al. Sulphasalazine accelerates apoptosis in neutrophils exposed to immune complex: role of caspase pathway. Clin. Exp. Pharmacol. Physiol. 36, 1132–1135 (2009).
Rossi, A. G. et al. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat. Med. 12, 1056–1064 (2006).
Bannenberg, G. L. et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J. Immunol. 174, 4345–4355 (2005).
Damazo, A. S., Yona, S., Flower, R. J., Perretti, M. & Oliani, S. M. Spatial and temporal profiles for anti-inflammatory gene expression in leukocytes during a resolving model of peritonitis. J. Immunol. 176, 4410–4418 (2006).
Serhan, C. N., Hamberg, M. & Samuelsson, B. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc. Natl Acad. Sci. USA 81, 5335–5339 (1984).
Romano, M. et al. Lipoxin synthase activity of human platelet 12-lipoxygenase. Biochem. J. 296, 127–133 (1993).
Lu, Y., Hong, S., Tjonahen, E. & Serhan, C. N. Mediator-lipidomics: databases and search algorithms for PUFA-derived mediators. J. Lipid Res. 46, 790–802 (2005).
Dalli, J. & Serhan, C. N. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 120, e60–e72 (2012).
Colas, R. A., Shinohara, M., Dalli, J., Chiang, N. & Serhan, C. N. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am. J. Physiol. Cell Physiol. 307, C39–C54 (2014).
Arnardottir, H., Orr, S. K., Dalli, J. & Serhan, C. N. Human milk proresolving mediators stimulate resolution of acute inflammation. Mucosal Immunol. 9, 757–766 (2016).
Sasaki, A. et al. Determination of ω-6 and ω-3 PUFA metabolites in human urine samples using UPLC/MS/MS. Anal. Bioanal. Chem. 407, 1625–1639 (2015).
Markworth, J. F. et al. Divergent shifts in lipid mediator profile following supplementation with n-3 docosapentaenoic acid and eicosapentaenoic acid. FASEB J. 30, 3714–3725 (2016).
Weiss, G. A. et al. High levels of anti-inflammatory and pro-resolving lipid mediators lipoxins and resolvins and declining docosahexaenoic acid levels in human milk during the first month of lactation. Lipids Health Dis. 12, 89 (2013).
Barden, A. E. et al. Specialised pro-resolving mediators of inflammation in inflammatory arthritis. Prostaglandins Leukot. Essent. Fatty Acids 107, 24–29 (2016).
Jones, M. L. et al. Maternal dietary omega-3 fatty acid intake increases resolvin and protectin levels in the rat placenta. J. Lipid Res. 54, 2247–2254 (2013).
Morita, M. et al. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell 153, 112–125 (2013).
Miles, E. A. & Calder, P. C. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br. J. Nutr. 107 (Suppl. 2), S171–S184 (2012).
Kronke, G. et al. 12/15-lipoxygenase counteracts inflammation and tissue damage in arthritis. J. Immunol. 183, 3383–3389 (2009).
Dufton, N. et al. Anti-inflammatory role of the murine formyl-peptide receptor 2: ligand-specific effects on leukocyte responses and experimental inflammation. J. Immunol. 184, 2611–2619 (2010).
Blaho, V. A., Zhang, Y., Hughes-Hanks, J. M. & Brown, C. R. 5-lipoxygenase-deficient mice infected with Borrelia burgdorferi develop persistent arthritis. J. Immunol. 186, 3076–3084 (2011).
Chan, M. M. & Moore, A. R. Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase-2 inhibition and restored by prostaglandin E2-mediated lipoxin A4 production. J. Immunol. 184, 6418–6426 (2010).
Gheorghe, K. R. et al. Expression of 5-lipoxygenase and 15-lipoxygenase in rheumatoid arthritis synovium and effects of intraarticular glucocorticoids. Arthritis Res. Ther. 11, R83 (2009).
Chabane, N. et al. Human articular chondrocytes express 15-lipoxygenase-1 and -2: potential role in osteoarthritis. Arthritis Res. Ther. 11, R44 (2009).
Hashimoto, A. et al. Antiinflammatory mediator lipoxin A4 and its receptor in synovitis of patients with rheumatoid arthritis. J. Rheumatol. 34, 2144–2153 (2007).
Norling, L. V. et al. Proresolving and cartilage-protective actions of resolvin D1 in inflammatory arthritis. JCI Insight 1, e85922 (2016).
Giera, M. et al. Lipid and lipid mediator profiling of human synovial fluid in rheumatoid arthritis patients by means of LC-MS/MS. Biochim. Biophys. Acta 1821, 1415–1424 (2012).
Lima-Garcia, J. F. et al. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br. J. Pharmacol. 164, 278–293 (2011).
Borea, P. A., Gessi, S., Merighi, S. & Varani, K. Adenosine as a multi-signalling guardian angel in human diseases: when, where and how does it exert its protective effects? Trends Pharmacol. Sci. 37, 419–434 (2016).
Chan, E. S. & Cronstein, B. N. Methotrexate — how does it really work? Nat. Rev. Rheumatol. 6, 175–178 (2010).
Peres, R. S. et al. Low expression of CD39 on regulatory T cells as a biomarker for resistance to methotrexate therapy in rheumatoid arthritis. Proc. Natl Acad. Sci. USA 112, 2509–2514 (2015).
Siebert, S., Tsoukas, A., Robertson, J. & McInnes, I. Cytokines as therapeutic targets in rheumatoid arthritis and other inflammatory diseases. Pharmacol. Rev. 67, 280–309 (2015).
Norling, L. V. & Perretti, M. Control of myeloid cell trafficking in resolution. J. Innate Immun. 5, 367–376 (2013).
Flogel, U. et al. Selective activation of adenosine A2A receptors on immune cells by a CD73-dependent prodrug suppresses joint inflammation in experimental rheumatoid arthritis. Sci. Transl Med. 4, 146ra108 (2012).
Mediero, A. et al. Adenosine A2A receptor activation prevents wear particle-induced osteolysis. Sci. Transl Med. 4, 135ra65 (2012).
Wallace, J. L., Ianaro, A., Flannigan, K. L. & Cirino, G. Gaseous mediators in resolution of inflammation. Semin. Immunol. 27, 227–233 (2015).
Ahluwalia, A. et al. Antiinflammatory activity of soluble guanylate cyclase: cGMP-dependent down-regulation of P-selectin expression and leukocyte recruitment. Proc. Natl Acad. Sci. USA 101, 1386–1391 (2004).
Zanardo, R. C. et al. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 20, 2118–2120 (2006).
Brancaleone, V., Mitidieri, E., Flower, R. J., Cirino, G. & Perretti, M. Annexin A1 mediates hydrogen sulfide properties in the control of inflammation. J. Pharmacol. Exp. Ther. 351, 96–104 (2014).
Kloesch, B., Liszt, M. & Broell, J. H2S transiently blocks IL-6 expression in rheumatoid arthritic fibroblast-like synoviocytes and deactivates p44/42 mitogen-activated protein kinase. Cell Biol. Int. 34, 477–484 (2010).
Burguera, E. F., Vela-Anero, A., Magalhaes, J., Meijide-Failde, R. & Blanco, F. J. Effect of hydrogen sulfide sources on inflammation and catabolic markers on interleukin 1β-stimulated human articular chondrocytes. Osteoarthritis Cartilage 22, 1026–1035 (2014).
Dief, A. E., Mostafa, D. K., Sharara, G. M. & Zeitoun, T. H. Hydrogen sulfide releasing naproxen offers better anti-inflammatory and chondroprotective effect relative to naproxen in a rat model of zymosan induced arthritis. Eur. Rev. Med. Pharmacol. Sci. 19, 1537–1546 (2015).
Chiang, N. et al. Inhaled carbon monoxide accelerates resolution of inflammation via unique proresolving mediator-heme oxygenase-1 circuits. J. Immunol. 190, 6378–6388 (2013).
Shinohara, M. et al. Cell–cell interactions and bronchoconstrictor eicosanoid reduction with inhaled carbon monoxide and resolvin D1. Am. J. Physiol. Lung Cell. Mol. Physiol. 307, L746–L757 (2014).
Daoussis, D., Antonopoulos, I. & Andonopoulos, A. P. ACTH as a treatment for acute crystal-induced arthritis: update on clinical evidence and mechanisms of action. Semin. Arthritis Rheum. 43, 648–653 (2014).
Gutman, A. B. & Yu, T. F. Effects of adrenocorticotropic hormone (ACTH) in gout. Am. J. Med. 9, 24–30 (1950).
Hench, P. S. et al. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone; compound E) and of pituitary adrenocorticotropic hormone on rheumatoid arthritis. Proc. Staff Meet. Mayo Clin. 24, 181–197 (1949).
Montero-Melendez, T. ACTH: The forgotten therapy. Semin. Immunol. 27, 216–226 (2015).
Bohm, M. & Grassel, S. Role of proopiomelanocortin-derived peptides and their receptors in the osteoarticular system: from basic to translational research. Endocr. Rev. 33, 623–651 (2012).
Getting, S. J., Christian, H. C., Flower, R. J. & Perretti, M. Activation of melanocortin type 3 receptor as a molecular mechanism for adrenocorticotropic hormone efficacy in gouty arthritis. Arthritis Rheum. 46, 2765–2775 (2002).
Montero-Melendez, T. et al. Association between periodontal disease and inflammatory arthritis reveals modulatory functions by melanocortin receptor type 3. Am. J. Pathol. 184, 2333–2341 (2014).
Patel, H. B. et al. Anti-inflammatory and antiosteoclastogenesis properties of endogenous melanocortin receptor type 3 in experimental arthritis. FASEB J. 24, 4835–4843 (2010).
Grassel, S. et al. The melanocortin system in articular chondrocytes: melanocortin receptors, pro-opiomelanocortin, precursor proteases, and a regulatory effect of α-melanocyte-stimulating hormone on proinflammatory cytokines and extracellular matrix components. Arthritis Rheum. 60, 3017–3027 (2009).
Kaneva, M. K. et al. Melanocortin peptides protect chondrocytes from mechanically induced cartilage injury. Biochem. Pharmacol. 92, 336–347 (2014).
Muffley, L. A., Zhu, K. Q., Engrav, L. H., Gibran, N. S. & Hocking, A. M. Spatial and temporal localization of the melanocortin 1 receptor and its ligand α-melanocyte-stimulating hormone during cutaneous wound repair. J. Histochem. Cytochem. 59, 278–288 (2011).
Perretti, M. & D'Acquisto, F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 9, 62–70 (2009).
Drechsler, M. et al. Annexin A1 counteracts chemokine-induced arterial myeloid cell recruitment. Circ. Res. 116, 827–835 (2015).
Montero-Melendez, T., Dalli, J. & Perretti, M. Gene expression signature-based approach identifies a pro-resolving mechanism of action for histone deacetylase inhibitors. Cell Death Differ. 20, 567–575 (2013).
Locatelli, I. et al. Endogenous annexin A1 is a novel protective determinant in nonalcoholic steatohepatitis in mice. Hepatology 60, 531–544 (2014).
Fredman, G. et al. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci. Transl Med. 7, 275ra20 (2015).
Yang, Y. H. et al. Modulation of inflammation and response to dexamethasone by annexin 1 in antigen-induced arthritis. Arthritis Rheum. 50, 976–984 (2004).
Patel, H. B. et al. The impact of endogenous annexin A1 on glucocorticoid control of inflammatory arthritis. Ann. Rheum. Dis. 71, 1872–1880 (2012).
Sapolsky, R. M., Romero, L. M. & Munck, A. U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89 (2000).
Iqbal, A. J. et al. Endogenous galectin-1 and acute inflammation: emerging notion of a galectin-9 pro-resolving effect. Am. J. Pathol. 178, 1201–1209 (2011).
Ilarregui, J. M. et al. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat. Immunol. 10, 981–991 (2009).
Chiang, N. et al. Anesthetics impact the resolution of inflammation. PLoS ONE 3, e1879 (2008).
Thiemann, S., Man, J. H., Chang, M. H., Lee, B. & Baum, L. G. Galectin-1 regulates tissue exit of specific dendritic cell populations. J. Biol. Chem. 290, 22662–22677 (2015).
Forsman, H. et al. Galectin 3 aggravates joint inflammation and destruction in antigen-induced arthritis. Arthritis Rheum. 63, 445–454 (2011).
Arikawa, T. et al. Galectin-9 ameliorates immune complex-induced arthritis by regulating FcγR expression on macrophages. Clin. Immunol. 133, 382–392 (2009).
Rabinovich, G. A. et al. Recombinant galectin-1 and its genetic delivery suppress collagen-induced arthritis via T cell apoptosis. J. Exp. Med. 190, 385–398 (1999).
Iqbal, A. J. et al. Endogenous galectin-1 exerts tonic inhibition on experimental arthritis. J. Immunol. 191, 171–177 (2013).
Seki, M. et al. Beneficial effect of galectin 9 on rheumatoid arthritis by induction of apoptosis of synovial fibroblasts. Arthritis Rheum. 56, 3968–3976 (2007).
Seki, M. et al. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin. Immunol. 127, 78–88 (2008).
Naylor, A. J., Filer, A. & Buckley, C. D. The role of stromal cells in the persistence of chronic inflammation. Clin. Exp. Immunol. 171, 30–35 (2013).
Harjacek, M. et al. Expression of galectins-1 and -3 correlates with defective mononuclear cell apoptosis in patients with juvenile idiopathic arthritis. J. Rheumatol. 28, 1914–1922 (2001).
Xibille-Friedmann, D., Bustos Rivera-Bahena, C., Rojas-Serrano, J., Burgos-Vargas, R. & Montiel-Hernandez, J. L. A decrease in galectin-1 (Gal-1) levels correlates with an increase in anti-Gal-1 antibodies at the synovial level in patients with rheumatoid arthritis. Scand. J. Rheumatol. 42, 102–107 (2013).
Nishi, N., Itoh, A., Shoji, H., Miyanaka, H. & Nakamura, T. Galectin-8 and galectin-9 are novel substrates for thrombin. Glycobiology 16, 15C–20C (2006).
Schall, T. J. & Proudfoot, A. E. Overcoming hurdles in developing successful drugs targeting chemokine receptors. Nat. Rev. Immunol. 11, 355–363 (2011).
Wells, T. N., Power, C. A., Shaw, J. P. & Proudfoot, A. E. Chemokine blockers — therapeutics in the making? Trends Pharmacol. Sci. 27, 41–47 (2006).
Brancaleone, V. et al. A vasculo-protective circuit centered on lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 operative in murine microcirculation. Blood 122, 608–617 (2013).
Abdulnour, R. E. et al. Maresin 1 biosynthesis during platelet–neutrophil interactions is organ-protective. Proc. Natl Acad. Sci. USA 111, 16526–16531 (2014).
Souza, D. G. et al. The required role of endogenously produced lipoxin A4 and annexin-1 for the production of IL-10 and inflammatory hyporesponsiveness in mice. J. Immunol. 179, 8533–8543 (2007).
Chiang, N. et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484, 524–528 (2012).
Brancaleone, V. et al. Evidence for an anti-inflammatory loop centered on polymorphonuclear leukocyte formyl peptide receptor 2/lipoxin A4 receptor and operative in the inflamed microvasculature. J. Immunol. 186, 4905–4914 (2011).
Varani, K. et al. A2A and A3 adenosine receptor expression in rheumatoid arthritis: upregulation, inverse correlation with disease activity score and suppression of inflammatory cytokine and metalloproteinase release. Arthritis Res. Ther. 13, R197 (2011).
Perretti, M., Leroy, X., Bland, E. J. & Montero-Melendez, T. Resolution pharmacology: opportunities for therapeutic innovation in inflammation. Trends Pharmacol. Sci. 36, 737–755 (2015).
Morabito, L. et al. Methotrexate and sulfasalazine promote adenosine release by a mechanism that requires ecto-5′-nucleotidase-mediated conversion of adenine nucleotides. J. Clin. Invest. 101, 295–300 (1998).
Vago, J. P. et al. Proresolving actions of synthetic and natural protease inhibitors are mediated by annexin A1. J. Immunol. 196, 1922–1932 (2016).
Montero-Melendez, T. & Perretti, M. Connections in pharmacology: innovation serving translational medicine. Drug Discov. Today 19, 820–823 (2014).
Joosten, L. A. et al. Alpha-1-anti-trypsin-Fc fusion protein ameliorates gouty arthritis by reducing release and extracellular processing of IL-1β and by the induction of endogenous IL-1Ra. Ann. Rheum. Dis. 75, 1219–1227 (2016).
Orr, S. K., Colas, R. A., Dalli, J., Chiang, N. & Serhan, C. N. Proresolving actions of a new resolvin D1 analog mimetic qualifies as an immunoresolvent. Am. J. Physiol. Lung Cell. Mol. Physiol. 308, L904–L911 (2015).
Vessillier, S. et al. Molecular engineering of short half-life small peptides (VIP, alphaMSH and gamma(3)MSH) fused to latency-associated peptide results in improved anti-inflammatory therapeutics. Ann. Rheum. Dis. 71, 143–149 (2012).
Cooray, S. N. et al. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc. Natl Acad. Sci. USA 110, 18232–18237 (2013).
Bozinovski, S. et al. Serum amyloid A opposes lipoxin A4 to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proc. Natl Acad. Sci. USA 109, 935–940 (2012).
Montero-Melendez, T., Gobbetti, T., Cooray, S. N., Jonassen, T. E. & Perretti, M. Biased agonism as a novel strategy to harness the proresolving properties of melanocortin receptors without eliciting melanogenic effects. J. Immunol. 194, 3381–3388 (2015).
Yanez-Mo, M. et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 4, 27066 (2015).
Dalli, J. et al. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood 112, 2512–2519 (2008).
Gasser, O. & Schifferli, J. A. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood 104, 2543–2548 (2004).
Norling, L. V. et al. Cutting edge: humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J. Immunol. 186, 5543–5547 (2011).
Van Dyke, T. E. et al. Proresolving nanomedicines activate bone regeneration in periodontitis. J. Dent. Res. 94, 148–156 (2015).
Headland, S. E. et al. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci. Transl Med. 7, 315ra190 (2015).
Leoni, G. et al. Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J. Clin. Invest. 125, 1215–1227 (2015).
Dalli, J., Chiang, N. & Serhan, C. N. Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections. Nat. Med. 21, 1071–1075 (2015).
Lee, C. R. & Zeldin, D. C. Resolvin infectious inflammation by targeting the host response. N. Engl. J. Med. 373, 2183–2185 (2015).
Wallace, J. L. & Wang, R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 14, 329–345 (2015).
Robertson, J., Peters, M. J., McInnes, I. B. & Sattar, N. Changes in lipid levels with inflammation and therapy in RA: a maturing paradigm. Nat. Rev. Rheumatol. 9, 513–523 (2013).
Gabay, C. et al. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann. Rheum. Dis. 75, 1806–1812 (2016).
Dalli, J. et al. Microparticle alpha-2-macroglobulin enhances pro-resolving responses and promotes survival in sepsis. EMBO Mol. Med. 6, 27–42 (2014).
Dalli, J. C. et al. Human sepsis eicosanoid and proresolving lipid mediator temporal profiles: correlations with survival and clinical outcome. Crit. Care Med. http://dx.doi.org/10.1097/CCM.0000000000002014 (2016).
Nadkarni, S. et al. Investigational analysis reveals a potential role for neutrophils in giant-cell arteritis disease progression. Circ. Res. 114, 242–248 (2014).
D'Acquisto, F. et al. Annexin-1 modulates T-cell activation and differentiation. Blood 109, 1095–1102 (2007).
Newson, J. et al. Resolution of acute inflammation bridges the gap between innate and adaptive immunity. Blood 124, 1748–1764 (2014).
Norling, L. V., Sampaio, A. L., Cooper, D. & Perretti, M. Inhibitory control of endothelial galectin-1 on in vitro and in vivo lymphocyte trafficking. FASEB J. 22, 682–690 (2008).
He, J. & Baum, L. G. Endothelial cell expression of galectin-1 induced by prostate cancer cells inhibits T-cell transendothelial migration. Lab. Invest. 86, 578–590 (2006).
Cooper, D., Norling, L. V. & Perretti, M. Novel insights into the inhibitory effects of galectin-1 on neutrophil recruitment under flow. J. Leukoc. Biol. 83, 1459–1466 (2008).
Dias-Baruffi, M. et al. Dimeric galectin-1 induces surface exposure of phosphatidylserine and phagocytic recognition of leukocytes without inducing apoptosis. J. Biol. Chem. 278, 41282–41293 (2003).
Cedeno-Laurent, F. et al. Development of a nascent galectin-1 chimeric molecule for studying the role of leukocyte galectin-1 ligands and immune disease modulation. J. Immunol. 185, 4659–4672 (2010).
Perillo, N. L., Pace, K. E., Seilhamer, J. J. & Baum, L. G. Apoptosis of T cells mediated by galectin-1. Nature 378, 736–739 (1995).
Zhu, C. et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 6, 1245–1252 (2005).
Rostoker, R. et al. Galectin-1 induces 12/15-lipoxygenase expression in murine macrophages and favors their conversion toward a pro-resolving phenotype. Prostaglandins Other Lipid Mediat. 107, 85–94 (2013).
Chavele, K. M. & Ehrenstein, M. R. Regulatory T-cells in systemic lupus erythematosus and rheumatoid arthritis. FEBS Lett. 585, 3603–3610 (2011).
Garin, M. I. et al. Galectin-1: a key effector of regulation mediated by CD4+CD25+ T cells. Blood 109, 2058–2065 (2007).
Wu, C. et al. Galectin-9–CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity 41, 270–282 (2014).
Birnbaum, Y. et al. Augmentation of myocardial production of 15-epi-lipoxin-A4 by pioglitazone and atorvastatin in the rat. Circulation 114, 929–935 (2006).
Campos-Estrada, C. et al. Simvastatin and benznidazole-mediated prevention of Trypanosoma cruzi-induced endothelial activation: role of 15-epi-lipoxin A4 in the action of simvastatin. PLoS Negl. Trop. Dis. 9, e0003770 (2015).
Planaguma, A. et al. Lovastatin decreases acute mucosal inflammation via 15-epi-lipoxin A4 . Mucosal Immunol. 3, 270–279 (2010).
Acknowledgements
The authors received funding support from the Wellcome Trust Programme (grant 086867/Z/08/Z to M.P.), the UK Medical Research Council (grant MR/K013068/1 to M.P.) and Arthritis Research UK (Career Progression Fellowship 20387 to D.C. and Career Development Fellowship 19909 to L.V.N.). J. D. received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement No 677542). J.D. is also supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (grant 107613/Z/15/Z). This work forms part of the research themes contributing to the translational research portfolio of Barts and the London Cardiovascular Biomedical Research Unit, which is supported and funded by the National Institutes of Health Research.
Author information
Authors and Affiliations
Contributions
M.P. researched data for article. All authors made a substantial contribution to discussion of content and writing the article. D.C., J.D. and L.V.N. reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M.P. is an adviser to Palatin Technologies and a member of the Scientific Advisory Board of Antibe Therapeutics. The other authors declare they have no competing interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Perretti, M., Cooper, D., Dalli, J. et al. Immune resolution mechanisms in inflammatory arthritis. Nat Rev Rheumatol 13, 87–99 (2017). https://doi.org/10.1038/nrrheum.2016.193
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2016.193
This article is cited by
-
Resolvin T4 enhances macrophage cholesterol efflux to reduce vascular disease
Nature Communications (2024)
-
Formyl peptide receptor 2 as a potential therapeutic target for inflammatory bowel disease
Acta Pharmacologica Sinica (2023)
-
From Inflammation to Resolution: Specialized Pro-resolving Mediators in Posttraumatic Osteoarthritis
Current Osteoporosis Reports (2023)
-
Activation of GPR37 in macrophages confers protection against infection-induced sepsis and pain-like behaviour in mice
Nature Communications (2021)
-
Blood pro-resolving mediators are linked with synovial pathology and are predictive of DMARD responsiveness in rheumatoid arthritis
Nature Communications (2020)