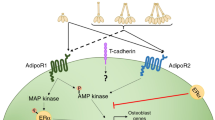
Key Points
-
Factors derived from adipose tissue contribute to inflammation as well as to alterations to cartilage and bone
-
Expression of adipokines is elevated in rheumatic diseases, both systemically and locally at sites of bone damage
-
Adipokines not only have a proinflammatory effect in chronic inflammatory arthritis, but also affect bone remodelling in degenerative joint diseases such as osteoarthritis
-
Adipokines are potential biomarkers of disease owing to their association with progression of bone erosions and osteophyte formation
-
Targeting adipokine dysregulation presents future therapeutic options to modulate not only the immune response, but also cartilage and bone remodelling
Abstract
Adipose tissue secretes highly bioactive factors, the adipokines. Systemic levels of adipokines are often altered in the presence of inflammation. In turn, adipokines affect different tissues and cells systemically as well as locally, contributing to immunomodulatory and bone remodelling mechanisms. The role of adipokines has been evaluated in chronic inflammatory diseases, such as rheumatoid arthritis, as well as in primarily degenerative joint diseases, such as osteoarthritis, particularly with regard to their levels of expression and their effects on joint tissues including synovial membrane, cartilage and bone. Distinct adipokines have been found to modulate matrix remodelling as well as inflammatory responses. In this Review, we summarize current knowledge relating to adipokines in rheumatic diseases, with a particular focus on the effects of adipokines on bone remodelling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Tilg, H. & Moschen, A. R. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 6, 772–783 (2006).
Gomez, R. et al. What's new in our understanding of the role of adipokines in rheumatic diseases? Nat. Rev. Rheumatol. 7, 528–536 (2011).
Neumann, E., Frommer, K. W., Vasile, M. & Muller-Ladner, U. Adipocytokines as driving forces in rheumatoid arthritis and related inflammatory diseases? Arthritis Rheum. 63, 1159–1169 (2011).
Schäffler, A. et al. Adipocytokines in synovial fluid. JAMA 290, 1709–1710 (2003).
Klein-Wieringa, I. R. et al. The infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype. Ann. Rheum. Dis. 70, 851–857 (2011).
van der Kraan, P. M. & van den Berg, W. B. Osteophytes: relevance and biology. Osteoarthritis Cartilage 15, 237–244 (2007).
Giganti, M. G. et al. Fracture healing: from basic science to role of nutrition. Front. Biosci. (Landmark Ed.) 19, 1162–1175 (2014).
Neumann, E. & Schett, G. Bone metabolism: molecular mechanisms. Z. Rheumatol. 66, 286–289 (in German) (2007).
Baum, R. & Gravallese, E. M. Impact of inflammation on the osteoblast in rheumatic diseases. Curr. Osteoporos. Rep. 12, 9–16 (2014).
Jung, S. M., Kim, K. W., Yang, C. W., Park, S. H. & Ju, J. H. Cytokine-mediated bone destruction in rheumatoid arthritis. J. Immunol. Res. 2014, 263625 (2014).
Swales, C. & Sabokbar, A. Cellular and molecular mechanisms of bone damage and repair in inflammatory arthritis. Drug Discov. Today 19, 1178–1185 (2014).
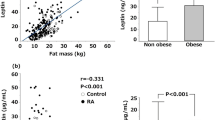
Karvonen-Gutierrez, C. A., Harlow, S. D., Jacobson, J., Mancuso, P. & Jiang, Y. The relationship between longitudinal serum leptin measures and measures of magnetic resonance imaging-assessed knee joint damage in a population of mid-life women. Ann. Rheum. Dis. 73, 883–889 (2014).
Scotece, M. et al. Leptin in joint and bone diseases: new insights. Curr. Med. Chem. 20, 3416–3425 (2013).
Perruccio, A. V., Mahomed, N. N., Chandran, V. & Gandhi, R. Plasma adipokine levels and their association with overall burden of painful joints among individuals with hip and knee osteoarthritis. J. Rheumatol. 41, 334–337 (2014).
Durmus, D., Alayli, G., Aliyazicioglu, Y., Buyukakincak, O. & Canturk, F. Effects of glucosamine sulfate and exercise therapy on serum leptin levels in patients with knee osteoarthritis: preliminary results of randomized controlled clinical trial. Rheumatol. Int. 33, 593–599 (2013).
Staikos, C. et al. The association of adipokine levels in plasma and synovial fluid with the severity of knee osteoarthritis. Rheumatology (Oxford) 52, 1077–1083 (2013).
Tian, G. et al. Increased leptin levels in patients with rheumatoid arthritis: a meta-analysis. Ir. J. Med. Sci. 183, 659–666 (2014).
Stavropoulos-Kalinoglou, A. et al. Obesity in rheumatoid arthritis. Rheumatology (Oxford) 50, 450–462 (2011).
Gomez, R. et al. Adiponectin and leptin increase IL-8 production in human chondrocytes. Ann. Rheum. Dis. 70, 2052–2054 (2011).
Conde, J. et al. Adiponectin and leptin induce VCAM-1 expression in human and murine chondrocytes. PLoS ONE 7, e52533 (2012).
Yaykasli, K. O. et al. Leptin induces ADAMTS-4, ADAMTS-5, and ADAMTS-9 genes expression by mitogen-activated protein kinases and NF-κB signaling pathways in human chondrocytes. Cell Biol. Int. 39, 104–112 (2014).
Muraoka, S., Kusunoki, N., Takahashi, H., Tsuchiya, K. & Kawai, S. Leptin stimulates interleukin-6 production via Janus kinase 2/signal transducer and activator of transcription 3 in rheumatoid synovial fibroblasts. Clin. Exp. Rheumatol. 31, 589–595 (2013).
Bouvard, B. et al. Hypoxia and vitamin D differently contribute to leptin and dickkopf-related protein 2 production in human osteoarthritic subchondral bone osteoblasts. Arthritis Res. Ther. 16, 459 (2014).
Yang, W. H. et al. Leptin induces oncostatin M production in osteoblasts by downregulating miR-93 through the Akt signaling pathway. Int. J. Mol. Sci. 15, 15778–15790 (2014).
Liu, G. Y. et al. Leptin promotes the osteoblastic differentiation of vascular smooth muscle cells from female mice by increasing RANKL expression. Endocrinology 155, 558–567 (2014).
Cirmanova, V., Bayer, M., Starka, L. & Zajickova, K. The effect of leptin on bone: an evolving concept of action. Physiol. Res. 57, S143–S151 (2008).
Iwata, M. et al. Initial responses of articular tissues in a murine high-fat diet-induced osteoarthritis model: pivotal role of the IPFP as a cytokine fountain. PLoS ONE 8, e60706 (2013).
Laiguillon, M. C. et al. Expression and function of visfatin (Nampt), an adipokine-enzyme involved in inflammatory pathways of osteoarthritis. Arthritis Res. Ther. 16, R38 (2014).
Li, Y. et al. Nicotinamide phosphoribosyltransferase (Nampt) affects the lineage fate determination of mesenchymal stem cells: a possible cause for reduced osteogenesis and increased adipogenesis in older individuals. J. Bone Miner. Res. 26, 2656–2664 (2013).
Moschen, A. R., Geiger, S., Gerner, R. & Tilg, H. Pre-B cell colony enhancing factor/NAMPT/visfatin and its role in inflammation-related bone disease. Mutat. Res. 690, 95–101 (2010).
Venkateshaiah, S. U. et al. NAMPT/PBEF1 enzymatic activity is indispensable for myeloma cell growth and osteoclast activity. Exp. Hematol. 41, 547–557.e2 (2013).
Jiang, L., Bao, J., Zhou, X., Xiong, Y. & Wu, L. Increased serum levels and chondrocyte expression of nesfatin-1 in patients with osteoarthritis and its relation with BMI, hsCRP, and IL-18. Mediators Inflamm. 2013, 631251 (2013).
Lago, R. et al. A new player in cartilage homeostasis: adiponectin induces nitric oxide synthase type II and pro-inflammatory cytokines in chondrocytes. Osteoarthritis Cartilage 16, 1101–1109 (2008).
Kanazawa, I. et al. Adiponectin and AMP kinase activator stimulate proliferation, differentiation, and mineralization of osteoblastic MC3T3-E1 cells. BMC Cell Biol. 8, 51 (2007).
Shinoda, Y. et al. Regulation of bone formation by adiponectin through autocrine/paracrine and endocrine pathways. J. Cell. Biochem. 99, 196–208 (2006).
Lin, Y. Y. et al. Adiponectin receptor 1 regulates bone formation and osteoblast differentiation by GSK-3β/β-catenin signaling in mice. Bone 64, 147–154 (2014).
Chen, T., Wu, Y. W., Lu, H., Guo, Y. & Tang, Z. H. Adiponectin enhances osteogenic differentiation in human adipose-derived stem cells by activating the APPL1–AMPK signaling pathway. Biochem. Biophys. Res. Commun. 461, 237–242 (2015).
Pacheco-Pantoja, E. L., Fraser, W. D., Wilson, P. J. & Gallagher, J. A. Differential effects of adiponectin in osteoblast-like cells. J. Recept. Signal Transduct. Res. 34, 351–360 (2014).
Lee, Y. A. et al. The role of adiponectin in the production of IL-6, IL-8, VEGF and MMPs in human endothelial cells and osteoblasts: implications for arthritic joints. Exp. Mol. Med. 46, e72 (2014).
Oshima, K. et al. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem. Biophys. Res. Commun. 331, 520–526 (2005).
Williams, G. A. et al. In vitro and in vivo effects of adiponectin on bone. Endocrinology 150, 3603–3610 (2009).
Yamaguchi, N. et al. Adiponectin inhibits induction of TNF-α/RANKL-stimulated NFATc1 via the AMPK signaling. FEBS Lett. 582, 451–456 (2008).
Meyer, M. et al. Serum level of adiponectin is a surrogate independent biomarker of radiographic disease progression in early rheumatoid arthritis: results from the ESPOIR cohort. Arthritis Res. Ther. 15, R210 (2013).
Kim, K. S. et al. Serum adipokine levels in rheumatoid arthritis patients and their contributions to the resistance to treatment. Mol. Med. Rep. 9, 255–260 (2014).
Andres Cerezo, L. et al. The level of fatty acid-binding protein 4, a novel adipokine, is increased in rheumatoid arthritis and correlates with serum cholesterol levels. Cytokine 64, 441–447 (2013).
Makrilakis, K., Fragiadaki, K., Smith, J., Sfikakis, P. P. & Kitas, G. D. Interrelated reduction of chemerin and plasminogen activator inhibitor-1 serum levels in rheumatoid arthritis after interleukin-6 receptor blockade. Clin. Rheumatol. 34, 419–427 (2014).
Muruganandan, S., Roman, A. A. & Sinal, C. J. Role of chemerin/CMKLR1 signaling in adipogenesis and osteoblastogenesis of bone marrow stem cells. J. Bone Miner. Res. 25, 222–234 (2010).
Swales, C., Athanasou, N. A. & Knowles, H. J. Angiopoietin-like 4 is over-expressed in rheumatoid arthritis patients: association with pathological bone resorption. PLoS ONE 9, e109524 (2014).
Murayama, M. A. et al. CTRP3 plays an important role in the development of collagen-induced arthritis in mice. Biochem. Biophys. Res. Commun. 443, 42–48 (2014).
Dikbas, O. et al. Serum levels of visfatin, resistin and adiponectin in patients with psoriatic arthritis and associations with disease severity. Int. J. Rheum. Dis. http://dx.doi.org/10.1111/1756-185X.12444 (2014).
Eder, L. et al. Serum adipokines in patients with psoriatic arthritis and psoriasis alone and their correlation with disease activity. Ann. Rheum. Dis. 72, 1956–1961 (2013).
Syrbe, U. et al. Serum adipokine levels in patients with ankylosing spondylitis and their relationship to clinical parameters and radiographic spinal progression. Arthritis Rheumatol. 67, 678–685 (2015).
Miranda-Filloy, J. A. et al. Leptin and visfatin serum levels in non-diabetic ankylosing spondylitis patients undergoing TNF-α antagonist therapy. Clin. Exp. Rheumatol. 31, 538–545 (2013).
Genre, F. et al. Correlation between insulin resistance and serum ghrelin in non-diabetic ankylosing spondylitis patients undergoing anti-TNF-α therapy. Clin. Exp. Rheumatol. 31, 913–918 (2013).
Ma, C. et al. Genetic determination of the cellular basis of the ghrelin-dependent bone remodeling. Mol. Metab. 4, 175–185 (2015).
Conde, J. et al. Differential expression of adipokines in infrapatellar fat pad (IPFP) and synovium of osteoarthritis patients and healthy individuals. Ann. Rheum. Dis. 73, 631–633 (2014).
Conde, J. et al. Identification of novel adipokines in the joint. Differential expression in healthy and osteoarthritis tissues. PLoS ONE 10, e0123601 (2015).
Scotece, M. et al. NUCB2/nesfatin-1: a new adipokine expressed in human and murine chondrocytes with pro-inflammatory properties, an in vitro study. J. Orthop. Res. 32, 653–660 (2014).
Conde, J. et al. Expression and modulation of adipolin/C1qdc2: a novel adipokine in human and murine ATDC-5 chondrocyte cell line. Ann. Rheum. Dis. 72, 140–142 (2013).
Scotece, M. et al. Adipokines as drug targets in joint and bone disease. Drug Discov. Today 19, 241–258 (2013).
Fukuhara, A. et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science 307, 426–430 (2005).
Ehling, A. et al. The potential of adiponectin in driving arthritis. J. Immunol. 176, 4468–4478 (2006).
Choi, H. M. et al. Adiponectin may contribute to synovitis and joint destruction in rheumatoid arthritis by stimulating vascular endothelial growth factor, matrix metalloproteinase-1, and matrix metalloproteinase-13 expression in fibroblast-like synoviocytes more than proinflammatory mediators. Arthritis Res. Ther. 11, R161 (2009).
Kitahara, K., Kusunoki, N., Kakiuchi, T., Suguro, T. & Kawai, S. Adiponectin stimulates IL-8 production by rheumatoid synovial fibroblasts. Biochem. Biophys. Res. Commun. 378, 218–223 (2009).
Tang, C. H., Chiu, Y. C., Tan, T. W., Yang, R. S. & Fu, W. M. Adiponectin enhances IL-6 production in human synovial fibroblast via an AdipoR1 receptor, AMPK, 38, and NF-κB pathway. J. Immunol. 179, 5483–5492 (2007).
Ebina, K. et al. Adenovirus-mediated gene transfer of adiponectin reduces the severity of collagen-induced arthritis in mice. Biochem. Biophys. Res. Commun. 378, 186–191 (2009).
Lee, S. W., Kim, J. H., Park, M. C., Park, Y. B. & Lee, S. K. Adiponectin mitigates the severity of arthritis in mice with collagen-induced arthritis. Scand. J. Rheumatol. 37, 260–268 (2008).
Brentano, F. et al. Pre-B cell colony-enhancing factor/visfatin, a new marker of inflammation in rheumatoid arthritis with proinflammatory and matrix-degrading activities. Arthritis Rheum. 56, 2829–2839 (2007).
Moschen, A. R. et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J. Immunol. 178, 1748–1758 (2007).
Nowell, M. A. et al. Regulation of pre-B cell colony-enhancing factor by STAT-3-dependent interleukin-6 trans-signaling: implications in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 54, 2084–2095 (2006).
Busso, N. et al. Pharmacological inhibition of nicotinamide phosphoribosyltransferase/visfatin enzymatic activity identifies a new inflammatory pathway linked to NAD. PLoS ONE 3, e2267 (2008).
Schaffler, A. et al. Adipocytokines in synovial fluid. JAMA 290, 1709–1710 (2003).
Forsblad d'Elia, H., Pullerits, R., Carlsten, H. & Bokarewa, M. Resistin in serum is associated with higher levels of IL-1Ra in post-menopausal women with rheumatoid arthritis. Rheumatology (Oxford) 47, 1082–1087 (2008).
Migita, K. et al. The serum levels of resistin in rheumatoid arthritis patients. Clin. Exp. Rheumatol. 24, 698–701 (2006).
Bokarewa, M., Nagaev, I., Dahlberg, L., Smith, U. & Tarkowski, A. Resistin, an adipokine with potent proinflammatory properties. J. Immunol. 174, 5789–5795 (2005).
Silswal, N. et al. Human resistin stimulates the pro-inflammatory cytokines TNF-α and IL-12 in macrophages by NF-κB-dependent pathway. Biochem. Biophys. Res. Commun. 334, 1092–1101 (2005).
Gonzalez-Gay, M. A. et al. Anti-TNF-α therapy modulates resistin in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 26, 311–316 (2008).
Anders, H. J., Rihl, M., Heufelder, A., Loch, O. & Schattenkirchner, M. Leptin serum levels are not correlated with disease activity in patients with rheumatoid arthritis. Metabolism 48, 745–748 (1999).
Gunaydin, R. et al. Serum leptin levels in rheumatoid arthritis and relationship with disease activity. South. Med. J. 99, 1078–1083 (2006).
Toussirot, E. et al. Relationship between growth hormone–IGF-I–IGFBP-3 axis and serum leptin levels with bone mass and body composition in patients with rheumatoid arthritis. Rheumatology (Oxford) 44, 120–125 (2005).
Hizmetli, S., Kisa, M., Gokalp, N. & Bakici, M. Z. Are plasma and synovial fluid leptin levels correlated with disease activity in rheumatoid arthritis? Rheumatol. Int. 27, 335–338 (2007).
Lee, S. W., Park, M. C., Park, Y. B. & Lee, S. K. Measurement of the serum leptin level could assist disease activity monitoring in rheumatoid arthritis. Rheumatol. Int. 27, 537–540 (2007).
Palmer, G. & Gabay, C. A role for leptin in rheumatic diseases? Ann. Rheum. Dis. 62, 913–915 (2003).
Popa, C. et al. Markers of inflammation are negatively correlated with serum leptin in rheumatoid arthritis. Ann. Rheum. Dis. 64, 1195–1198 (2005).
Popa, C. et al. Circulating leptin and adiponectin concentrations during tumor necrosis factor blockade in patients with active rheumatoid arthritis. J. Rheumatol. 36, 724–730 (2009).
Harle, P., Sarzi-Puttini, P., Cutolo, M. & Straub, R. H. No change of serum levels of leptin and adiponectin during anti-tumour necrosis factor antibody treatment with adalimumab in patients with rheumatoid arthritis. Ann. Rheum. Dis. 65, 970–971 (2006).
Otero, M. et al. Leptin: a metabolic hormone that functions like a proinflammatory adipokine. Drug News Perspect. 19, 21–26 (2006).
Otero, M. et al. Towards a pro-inflammatory and immunomodulatory emerging role of leptin. Rheumatology (Oxford) 45, 944–950 (2006).
Otero, M. et al. Leptin, from fat to inflammation: old questions and new insights. FEBS Lett. 579, 295–301 (2005).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, made substantial contributions to discussions of content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Neumann, E., Junker, S., Schett, G. et al. Adipokines in bone disease. Nat Rev Rheumatol 12, 296–302 (2016). https://doi.org/10.1038/nrrheum.2016.49
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2016.49
This article is cited by
-
A novel visceral adiposity index predicts bone loss in female early rheumatoid arthritis patients detected by HR-pQCT
Scientific Reports (2023)
-
Temporal Relationship Between Insulin Resistance and Lipid Accumulation After Bariatric Surgery: a Multicenter Cohort Study
Obesity Surgery (2023)
-
Balneotherapy year in review 2021: focus on the mechanisms of action of balneotherapy in rheumatic diseases
Environmental Science and Pollution Research (2022)
-
Inflammaging and Osteoarthritis
Clinical Reviews in Allergy & Immunology (2022)
-
The association between overweight/obesity and vertebral fractures in older adults: a meta-analysis of observational studies
Osteoporosis International (2021)