Key Points
-
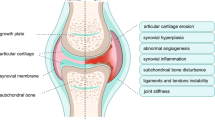
Osteoarthritis (OA) represents the failure of the joint as an organ
-
Synovitis is increasingly recognized as a characteristic of the OA joint, and its presence is associated with increased severity of symptoms, joint dysfunction, and cartilage loss
-
Studies in humans and animal models demonstrate a key role for chronic, low-grade inflammation in the pathogenesis of OA
-
Innate immune pathways, such as the complement and pattern-recognition receptor pathways, are pivotal to the inflammation in OA
-
Clinical trials are needed to determine whether anti-inflammatory therapeutics can prevent or slow disease progression in OA
Abstract
Osteoarthritis (OA) has long been viewed as a degenerative disease of cartilage, but accumulating evidence indicates that inflammation has a critical role in its pathogenesis. Furthermore, we now appreciate that OA pathogenesis involves not only breakdown of cartilage, but also remodelling of the underlying bone, formation of ectopic bone, hypertrophy of the joint capsule, and inflammation of the synovial lining. That is, OA is a disorder of the joint as a whole, with inflammation driving many pathologic changes. The inflammation in OA is distinct from that in rheumatoid arthritis and other autoimmune diseases: it is chronic, comparatively low-grade, and mediated primarily by the innate immune system. Current treatments for OA only control the symptoms, and none has been FDA-approved for the prevention or slowing of disease progression. However, increasing insight into the inflammatory underpinnings of OA holds promise for the development of new, disease-modifying therapies. Indeed, several anti-inflammatory therapies have shown promise in animal models of OA. Further work is needed to identify effective inhibitors of the low-grade inflammation in OA, and to determine whether therapies that target this inflammation can prevent or slow the development and progression of the disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lawrence, R. C. et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 58, 26–35 (2008).
Felson, D. T. Clinical practice. Osteoarthritis of the knee. N. Engl. J. Med. 354, 841–848 (2006).
Felson, D. T. et al. Osteoarthritis: new insights. Part 2: treatment approaches. Ann. Intern. Med. 133, 726–737 (2000).
Blagojevic, M., Jinks, C., Jeffery, A. & Jordan, K. P. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage 18, 24–33 (2010).
Zhuo, Q., Yang, W., Chen, J. & Wang, Y. Metabolic syndrome meets osteoarthritis. Nat. Rev. Rheumatol. 8, 729–737 (2012).
Loeser, R. F., Goldring, S. R., Scanzello, C. R. & Goldring, M. B. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 64, 1697–1707 (2012).
Sellam, J. & Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 6, 625–635 (2010).
Karsdal, M. A. et al. The coupling of bone and cartilage turnover in osteoarthritis: opportunities for bone antiresorptives and anabolics as potential treatments? Ann. Rheum. Dis. 73, 336–348 (2014).
Goldring, M. B. & Goldring, S. R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. NY Acad. Sci. 1192, 230–237 (2010).
Liu-Bryan, R. & Terkeltaub, R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 11, 35–44 (2015).
Brandt, K. D., Dieppe, P. & Radin, E. L. Commentary: is it useful to subset “primary” osteoarthritis? A critique based on evidence regarding the etiopathogenesis of osteoarthritis. Semin. Arthritis Rheum. 39, 81–95 (2009).
Guermazi, A. et al. Assessment of synovitis with contrast-enhanced MRI using a whole-joint semiquantitative scoring system in people with, or at high risk of, knee osteoarthritis: the MOST study. Ann. Rheum. Dis. 70, 805–811 (2011).
Ishijima, M. et al. Relationships between biomarkers of cartilage, bone, synovial metabolism and knee pain provide insights into the origins of pain in early knee osteoarthritis. Arthritis Res. Ther. 13, R22 (2011).
Pessler, F. et al. The synovitis of “non-inflammatory” orthopaedic arthropathies: a quantitative histological and immunohistochemical analysis. Ann. Rheum. Dis. 67, 1184–1187 (2008).
Scanzello, C. R. & Goldring, S. R. The role of synovitis in osteoarthritis pathogenesis. Bone 51, 249–257 (2012).
Goldring, M. B. & Goldring, S. R. Osteoarthritis. J. Cell. Physiol. 213, 626–634 (2007).
Bondeson, J. et al. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 62, 647–657 (2010).
Nettelbladt, E. & Sundblad, L. Protein patterns in synovial fluid and serum in rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 2, 144–151 (1959).
Sohn, D. H. et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res. Ther. 14, R7 (2012).
Gobezie, R. et al. High abundance synovial fluid proteome: distinct profiles in health and osteoarthritis. Arthritis Res. Ther. 9, R36 (2007).
Pelletier, J. P., Martel-Pelletier, J. & Abramson, S. B. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 44, 1237–1247 (2001).
Roemer, F. W. et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann. Rheum. Dis. 70, 1804–1809 (2011).
Hill, C. L. et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann. Rheum. Dis. 66, 1599–1603 (2007).
Torres, L. et al. The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthritis Cartilage 14, 1033–1040 (2006).
Baker, K. et al. Relation of synovitis to knee pain using contrast-enhanced MRIs. Ann. Rheum. Dis. 69, 1779–1783 (2010).
Scanzello, C. R. et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum. 63, 391–400 (2011).
Krasnokutsky, S. et al. Quantitative magnetic resonance imaging evidence of synovial proliferation is associated with radiographic severity of knee osteoarthritis. Arthritis Rheum. 63, 2983–2991 (2011).
Sowers, M., Karvonen-Gutierrez, C. A., Jacobson, J. A., Jiang, Y. & Yosef, M. Associations of anatomical measures from MRI with radiographically defined knee osteoarthritis score, pain, and physical functioning. J. Bone Joint Surg. Am. 93, 241–251 (2011).
Ayral, X., Pickering, E. H., Woodworth, T. G., Mackillop, N. & Dougados, M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis — results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage 13, 361–367 (2005).
Sokolove, J. & Lepus, C. M. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther. Adv. Musculoskeletal Dis. 5, 77–94 (2013).
Benito, M. J., Veale, D. J., FitzGerald, O., van den Berg, W. B. & Bresnihan, B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 64, 1263–1267 (2005).
Chevalier, X., Eymard, F. & Richette, P. Biologic agents in osteoarthritis: hopes and disappointments. Nat. Rev. Rheumatol. 9, 400–410 (2013).
Husa, M., Liu-Bryan, R. & Terkeltaub, R. Shifting HIFs in osteoarthritis. Nat. Med. 16, 641–644 (2010).
Glasson, S. S. In vivo osteoarthritis target validation utilizing genetically-modified mice. Curr. Drug Targets 8, 367–376 (2007).
van Lent, P. L. et al. Crucial role of synovial lining macrophages in the promotion of transforming growth factor β-mediated osteophyte formation. Arthritis Rheum. 50, 103–111 (2004).
Felson, D. T., Anderson, J. J., Naimark, A., Walker, A. M. & Meenan, R. F. Obesity and knee osteoarthritis. The Framingham Study. Ann. Intern. Med. 109, 18–24 (1988).
Berenbaum, F., Eymard, F. & Houard, X. Osteoarthritis, inflammation and obesity. Curr. Opin. Rheumatol. 25, 114–118 (2013).
You, T. & Nicklas, B. J. Chronic inflammation: role of adipose tissue and modulation by weight loss. Curr. Diabetes Rev. 2, 29–37 (2006).
Beavers, K. M. et al. Effects of total and regional fat loss on plasma CRP and IL-6 in overweight and obese, older adults with knee osteoarthritis. Osteoarthritis Cartilage 23, 249–256 (2015).
Vincent, H. K., Heywood, K., Connelly, J. & Hurley, R. W. Obesity and weight loss in the treatment and prevention of osteoarthritis. PM R. 4 (5 Suppl.), S59–S67 (2012).
Haseeb, A. & Haqqi, T. M. Immunopathogenesis of osteoarthritis. Clin. Immunol. 146, 185–196 (2013).
Scanzello, C. R., Plaas, A. & Crow, M. K. Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Current opinion in rheumatology 20, 565–572 (2008).
Krenn, V. et al. Grading of chronic synovitis — a histopathological grading system for molecular and diagnostic pathology. Pathol. Res. Pract. 198, 317–325 (2002).
Slansky, E. et al. Quantitative determination of the diagnostic accuracy of the synovitis score and its components. Histopathology 57, 436–443 (2010).
de Lange-Brokaar, B. J. et al. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage 20, 1484–1499 (2012).
Cohen, S. B. et al. A randomized, double-blind study of AMG 108 (a fully human monoclonal antibody to IL-1R1) in patients with osteoarthritis of the knee. Arthritis Res. Ther. 13, R125 (2011).
Magnano, M. D. et al. A pilot study of tumor necrosis factor inhibition in erosive/inflammatory osteoarthritis of the hands. J. Rheumatol. 34, 1323–1327 (2007).
Verbruggen, G., Wittoek, R., Vander Cruyssen, B. & Elewaut, D. Tumour necrosis factor blockade for the treatment of erosive osteoarthritis of the interphalangeal finger joints: a double blind, randomised trial on structure modification. Ann. Rheum. Dis. 71, 891–898 (2012).
Chevalier, X. et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 61, 344–352 (2009).
Orlowsky, E. W. & Kraus, V. B. The role of innate immunity in osteoarthritis: when our first line of defense goes on the offensive. J. Rheumatol. 42, 363–371 (2015).
Fearon, D. T. & Locksley, R. M. The instructive role of innate immunity in the acquired immune response. Science 272, 50–53 (1996).
Holers, V. M. & Thurman, J. M. The alternative pathway of complement in disease: opportunities for therapeutic targeting. Mol. Immunol. 41, 147–152 (2004).
Wang, Q. et al. Identification of a central role for complement in osteoarthritis. Nat. Med. 17, 1674–1679 (2011).
Rus, H., Cudrici, C. & Niculescu, F. The role of the complement system in innate immunity. Immunol. Res. 33, 103–112 (2005).
Song, W. C., Sarrias, M. R. & Lambris, J. D. Complement and innate immunity. Immunopharmacology 49, 187–198 (2000).
Happonen, K. E. et al. Regulation of complement by COMP allows for a novel molecular diagnostic principle in rheumatoid arthritis. Arthritis Rheum. 62, 3574–3783 (2010).
Sjoberg, A. P. et al. Short leucine-rich glycoproteins of the extracellular matrix display diverse patterns of complement interaction and activation. Mol. Immunol. 46, 830–839 (2009).
Sjoberg, A., Onnerfjord, P., Morgelin, M., Heinegard, D. & Blom, A. M. The extracellular matrix and inflammation: fibromodulin activates the classical pathway of complement by directly binding C1q. J. Biol. Chem. 280, 32301–32308 (2005).
Moreth, K., Iozzo, R. V. & Schaefer, L. Small leucine-rich proteoglycans orchestrate receptor crosstalk during inflammation. Cell Cycle 11, 2084–2091 (2012).
Rosenthal, A. K. Crystals, inflammation, and osteoarthritis. Curr. Opin. Rheumatol. 23, 170–173 (2011).
Sofat, N. Analysing the role of endogenous matrix molecules in the development of osteoarthritis. Int. J. Exp. Pathol. 90, 463–479 (2009).
Nasi, S. et al. Dispensable role of myeloid differentiation primary response gene 88 (MyD88) and MyD88-dependent Toll-like receptors (TLRs) in a murine model of osteoarthritis. Joint Bone Spine 81, 320–324 (2014).
Liu-Bryan, R. Synovium and the innate inflammatory network in osteoarthritis progression. Curr. Rheumatol. Rep. 15, 323 (2013).
Rosado, C. J. et al. A common fold mediates vertebrate defense and bacterial attack. Science 317, 1548–1551 (2007).
Tschopp, J., Masson, D. & Stanley, K. K. Structural/functional similarity between proteins involved in complement- and cytotoxic T-lymphocyte-mediated cytolysis. Nature 322, 831–834 (1986).
Bohana-Kashtan, O., Ziporen, L., Donin, N., Kraus, S. & Fishelson, Z. Cell signals transduced by complement. Mol. Immunol. 41, 583–597 (2004).
Cooke, T. D., Bennett, E. L. & Ohno, O. The deposition of immunoglobulins and complement in osteoarthritic cartilage. Int. Orthop. 4, 211–217 (1980).
Corvetta, A. et al. Terminal complement complex in synovial tissue from patients affected by rheumatoid arthritis, osteoarthritis and acute joint trauma. Clin. Exp. Rheumatol. 10, 433–438 (1992).
Bradley, K. et al. Synthesis of classical pathway complement components by chondrocytes. Immunology 88, 648–656 (1996).
Kemper, C. & Atkinson, J. P. T-Cell regulation: with complements from innate immunity. Nat. Rev. Immunol. 7, 9–18 (2007).
Lepus, C. M. et al. Brief report: carboxypeptidase B serves as a protective mediator in osteoarthritis. Arthritis Rheumatol. 66, 101–106 (2014).
Boffa, M. C., Burke, B. & Haudenschild, C. C. Preservation of thrombomodulin antigen on vascular and extravascular surfaces. J. Histochem. Cytochem. 35, 1267–1276 (1987).
McCachren, S. S., Diggs, J., Weinberg, J. B. & Dittman, W. A. Thrombomodulin expression by human blood monocytes and by human synovial tissue lining macrophages. Blood 78, 3128–3132 (1991).
Conway, E. M., Nowakowski, B. & Steiner-Mosonyi, M. Human neutrophils synthesize thrombomodulin that does not promote thrombin-dependent protein C activation. Blood 80, 1254–1263 (1992).
Leung, L. L., Myles, T., Nishimura, T., Song, J. J. & Robinson, W. H. Regulation of tissue inflammation by thrombin-activatable carboxypeptidase B (or TAFI). Mol. Immunol. 45, 4080–4083 (2008).
Sharif, S. A. et al. Thrombin-activatable carboxypeptidase B cleavage of osteopontin regulates neutrophil survival and synoviocyte binding in rheumatoid arthritis. Arthritis Rheum. 60, 2902–2912 (2009).
Benoit, M. E., Clarke, E. V., Morgado, P., Fraser, D. A. & Tenner, A. J. Complement protein C1q directs macrophage polarization and limits inflammasome activity during the uptake of apoptotic cells. J. Immunol. 188, 5682–5693 (2012).
Foell, D., Wittkowski, H. & Roth, J. Mechanisms of disease: a 'DAMP' view of inflammatory arthritis. Nat. Clin. Pract. Rheumatol. 3, 382–390 (2007).
Blom, A. B. et al. Crucial role of macrophages in matrix metalloproteinase-mediated cartilage destruction during experimental osteoarthritis: involvement of matrix metalloproteinase 3. Arthritis Rheum. 56, 147–157 (2007).
Bondeson, J. Activated synovial macrophages as targets for osteoarthritis drug therapy. Curr. Drug Targets 11, 576–585 (2010).
Irani, A. A., Schechter, N. M., Craig, S. S., DeBlois, G. & Schwartz, L. B. Two types of human mast cells that have distinct neutral protease compositions. Proc. Natl Acad. Sci. USA 83, 4464–4468 (1986).
Buckley, M. G., Gallagher, P. J. & Walls, A. F. Mast cell subpopulations in the synovial tissue of patients with osteoarthritis: selective increase in numbers of tryptase-positive, chymase-negative mast cells. J. Pathol. 186, 67–74 (1998).
Gotis-Graham, I. & McNeil, H. P. Mast cell responses in rheumatoid synovium. Association of the MCTC subset with matrix turnover and clinical progression. Arthritis Rheum. 40, 479–489 (1997).
Nakano, S. et al. Distinct expression of mast cell tryptase and protease activated receptor-2 in synovia of rheumatoid arthritis and osteoarthritis. Clin. Rheumatol. 26, 1284–1292 (2007).
Bridges, A. J. et al. Human synovial mast cell involvement in rheumatoid arthritis and osteoarthritis. Relationship to disease type, clinical activity, and antirheumatic therapy. Arthritis Rheum. 34, 1116–1124 (1991).
Gruber, B. et al. Characterization and functional studies of rheumatoid synovial mast cells. Activation by secretagogues, anti-IgE, and a histamine-releasing lymphokine. Arthritis Rheum. 29, 944–955 (1986).
Kopicky-Burd, J. A. et al. Characterization of human synovial mast cells. J. Rheumatol. 15, 1326–1333 (1988).
Cooke, T. D. Significance of immune complex deposits in osteoarthritic cartilage. J. Rheumatol. 14, 77–79 (1987).
Vargas, M. E., Watanabe, J., Singh, S. J., Robinson, W. H. & Barres, B. A. Endogenous antibodies promote rapid myelin clearance and effective axon regeneration after nerve injury. Proc. Natl Acad. Sci. USA 107, 11993–11998 (2010).
Kapoor, M., Martel-Pelletier, J., Lajeunesse, D., Pelletier, J. P. & Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 7, 33–42 (2011).
Goldring, M. B., Fukuo, K., Birkhead, J. R., Dudek, E. & Sandell, L. J. Transcriptional suppression by interleukin-1 and interferon-γ of type II collagen gene expression in human chondrocytes. J. Cell Biochem. 54, 85–99 (1994).
Saklatvala, J. Tumour necrosis factor α stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature 322, 547–549 (1986).
Attur, M. G., Patel, I. R., Patel, R. N., Abramson, S. B. & Amin, A. R. Autocrine production of IL-1β by human osteoarthritis-affected cartilage and differential regulation of endogenous nitric oxide, IL-6, prostaglandin E2, and IL-8. Proc. Assoc. Am. Physicians 110, 65–72 (1998).
Krzeski, P. et al. Development of musculoskeletal toxicity without clear benefit after administration of PG-116800, a matrix metalloproteinase inhibitor, to patients with knee osteoarthritis: a randomized, 12-month, double-blind, placebo-controlled study. Arthritis Res. Ther. 9, R109 (2007).
Catterall, J. B. & Cawston, T. E. Drugs in development: bisphosphonates and metalloproteinase inhibitors. Arthritis Res. Ther. 5, 12–24 (2003).
Clutterbuck, A. L., Asplin, K. E., Harris, P., Allaway, D. & Mobasheri, A. Targeting matrix metalloproteinases in inflammatory conditions. Curr. Drug Targets 10, 1245–1254 (2009).
Endres, M. et al. Chemokine profile of synovial fluid from normal, osteoarthritis and rheumatoid arthritis patients: CCL25, CXCL10 and XCL1 recruit human subchondral mesenchymal progenitor cells. Osteoarthritis Cartilage 18, 1458–1466 (2010).
Haringman, J. J., Smeets, T. J., Reinders-Blankert, P. & Tak, P. P. Chemokine and chemokine receptor expression in paired peripheral blood mononuclear cells and synovial tissue of patients with rheumatoid arthritis, osteoarthritis, and reactive arthritis. Ann. Rheum. Dis. 65, 294–300 (2006).
Borzi, R. M. et al. Human chondrocytes express functional chemokine receptors and release matrix-degrading enzymes in response to C-X-C and C-C chemokines. Arthritis Rheum. 43, 1734–1741 (2000).
Miller, R. J., Banisadr, G. & Bhattacharyya, B. J. CXCR4 signaling in the regulation of stem cell migration and development. J. Neuroimmunol. 198, 31–38 (2008).
Haringman, J. J., Ludikhuize, J. & Tak, P. P. Chemokines in joint disease: the key to inflammation? Ann. Rheum. Dis. 63, 1186–1194 (2004).
Shen, J., Li, S. & Chen, D. TGF-β signaling and the development of osteoarthritis. Bone Res. 2, 14002 (2014).
Blaney Davidson, E. N., van der Kraan, P. M. & van den Berg, W. B. TGF-β and osteoarthritis. Osteoarthritis Cartilage 15, 597–604 (2007).
Ellman, M. B. et al. Fibroblast growth factor control of cartilage homeostasis. J. Cell Biochem. 114, 735–742 (2013).
Haywood, L. et al. Inflammation and angiogenesis in osteoarthritis. Arthritis Rheum. 48, 2173–2177 (2003).
Prencipe, G. et al. Nerve growth factor downregulates inflammatory response in human monocytes through TrkA. J. Immunol. 192, 3345–3354 (2014).
Lane, N. E. et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N. Engl. J. Med. 363, 1521–1531 (2010).
Thysen, S., Luyten, F. P. & Lories, R. J. Targets, models and challenges in osteoarthritis research. Dis. Model. Mech. 8, 17–30 (2015).
Henrotin, Y., Pesesse, L. & Lambert, C. Targeting the synovial angiogenesis as a novel treatment approach to osteoarthritis. Ther. Adv. Musculoskeletal Dis. 6, 20–34 (2014).
de Boer, T. N. et al. Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthritis Cartilage 20, 846–853 (2012).
Kluzek, S., Newton, J. L. & Arden, N. K. Is osteoarthritis a metabolic disorder? Br. Med. Bull. 115, 111–121 (2015).
Malemud, C. J. Biologic basis of osteoarthritis: state of the evidence. Curr. Opin. Rheumatol. 27, 289–294 (2015).
Yusuf, E. et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann. Rheum. Dis. 69, 761–765 (2010).
Le Clanche, S., Bonnefont-Rousselot, D., Sari-Ali, E., Rannou, F. & Borderie, D. Inter-relations between osteoarthritis and metabolic syndrome: a common link? Biochimie 121, 238–252 (2016).
Conde, J. et al. Adipokines and osteoarthritis: novel molecules involved in the pathogenesis and progression of disease. Arthritis 2011, 203901 (2011).
Gomez, R. et al. What's new in our understanding of the role of adipokines in rheumatic diseases? Nat. Rev. Rheumatol. 7, 528–536 (2011).
Dumond, H. et al. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum. 48, 3118–3129 (2003).
Filkova, M. et al. Increased serum adiponectin levels in female patients with erosive compared with non-erosive osteoarthritis. Ann. Rheum. Dis. 68, 295–296 (2009).
Francin, P. J. et al. Association between adiponectin and cartilage degradation in human osteoarthritis. Osteoarthritis Cartilage 22, 519–526 (2014).
Liao, L., Chen, Y. & Wang, W. The current progress in understanding the molecular functions and mechanisms of visfatin in osteoarthritis. J. Bone Miner. Metab. http://dx.doi.org/10.1007/s00774-016-0743-1 (2016).
Koskinen, A., Vuolteenaho, K., Moilanen, T. & Moilanen, E. Resistin as a factor in osteoarthritis: synovial fluid resistin concentrations correlate positively with interleukin 6 and matrix metalloproteinases MMP-1 and MMP-3. Scand. J. Rheumatol. 43, 249–253 (2014).
Goldring, M. B. & Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 23, 471–478 (2011).
Jiang, L., Bao, J., Zhou, X., Xiong, Y. & Wu, L. Increased serum levels and chondrocyte expression of nesfatin-1 in patients with osteoarthritis and its relation with BMI, hsCRP, and IL-18. Mediators Inflamm. 2013, 631251 (2013).
Yang, S. et al. NAMPT (visfatin), a direct target of hypoxia-inducible factor-2α, is an essential catabolic regulator of osteoarthritis. Ann. Rheum. Dis. 74, 595–602 (2015).
Gegout, P. P., Francin, P. J., Mainard, D. & Presle, N. Adipokines in osteoarthritis: friends or foes of cartilage homeostasis? Joint Bone Spine 75, 669–671 (2008).
Presle, N. et al. Differential distribution of adipokines between serum and synovial fluid in patients with osteoarthritis. Contribution of joint tissues to their articular production. Osteoarthritis Cartilage 14, 690–695 (2006).
Martel-Pelletier, J., Pelletier, J. P. & Fahmi, H. Cyclooxygenase-2 and prostaglandins in articular tissues. Semin. Arthritis Rheum. 33, 155–167 (2003).
Wittenberg, R. H., Willburger, R. E., Kleemeyer, K. S. & Peskar, B. A. In vitro release of prostaglandins and leukotrienes from synovial tissue, cartilage, and bone in degenerative joint diseases. Arthritis Rheum. 36, 1444–1450 (1993).
Casale, T. B., Abbas, M. K. & Carolan, E. J. Degree of neutrophil chemotaxis is dependent upon the chemoattractant and barrier. Am. J. Respir. Cell. Mol. Biol. 7, 112–117 (1992).
He, W., Pelletier, J. P., Martel-Pelletier, J., Laufer, S. & Di Battista, J. A. Synthesis of interleukin 1β, tumor necrosis factor-α, and interstitial collagenase (MMP-1) is eicosanoid dependent in human osteoarthritis synovial membrane explants: interactions with antiinflammatory cytokines. J. Rheumatol. 29, 546–553 (2002).
Peters-Golden, M. & Henderson, W. R. Jr. Leukotrienes. N. Engl. J. Med. 357, 1841–1854 (2007).
Geng, Y., Blanco, F. J., Cornelisson, M. & Lotz, M. Regulation of cyclooxygenase-2 expression in normal human articular chondrocytes. J. Immunol. 155, 796–801 (1995).
Li, X. et al. Expression and regulation of microsomal prostaglandin E synthase-1 in human osteoarthritic cartilage and chondrocytes. J. Rheumatol. 32, 887–895 (2005).
Masuko-Hongo, K. et al. Up-regulation of microsomal prostaglandin E synthase 1 in osteoarthritic human cartilage: critical roles of the ERK-1/2 and p38 signaling pathways. Arthritis Rheum. 50, 2829–2838 (2004).
Kojima, F. et al. Membrane-associated prostaglandin E synthase-1 is upregulated by proinflammatory cytokines in chondrocytes from patients with osteoarthritis. Arthritis Res. Ther. 6, R355–R365 (2004).
Paredes, Y. et al. Study of the role of leukotriene B4 in abnormal function of human subchondral osteoarthritis osteoblasts: effects of cyclooxygenase and/or 5-lipoxygenase inhibition. Arthritis Rheum. 46, 1804–1812 (2002).
Scarpignato, C. et al. Safe prescribing of non-steroidal anti-inflammatory drugs in patients with osteoarthritis — an expert consensus addressing benefits as well as gastrointestinal and cardiovascular risks. BMC Med. 13, 55 (2015).
Steinhilber, D. & Hofmann, B. Recent advances in the search for novel 5-lipoxygenase inhibitors. Bas. Clin. Pharmacol. Toxicol. 114, 70–77 (2014).
Tonge, D. P., Pearson, M. J. & Jones, S. W. The hallmarks of osteoarthritis and the potential to develop personalised disease-modifying pharmacological therapeutics. Osteoarthritis Cartilage 22, 609–621 (2014).
Abramson, S. B. Osteoarthritis and nitric oxide. Osteoarthritis Cartilage 16 (Suppl. 2), S15–S20 (2008).
Palmer, R. M., Hickery, M. S., Charles, I. G., Moncada, S. & Bayliss, M. T. Induction of nitric oxide synthase in human chondrocytes. Biochem. Biophys. Res. Commun. 193, 398–405 (1993).
McInnes, I. B. et al. Production of nitric oxide in the synovial membrane of rheumatoid and osteoarthritis patients. J. Exp. Med. 184, 1519–1524 (1996).
Farrell, A. J., Blake, D. R., Palmer, R. M. & Moncada, S. Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann. Rheum. Dis. 51, 1219–1222 (1992).
Hashimoto, S., Takahashi, K., Amiel, D., Coutts, R. D. & Lotz, M. Chondrocyte apoptosis and nitric oxide production during experimentally induced osteoarthritis. Arthritis Rheum. 41, 1266–1274, http://dx.doi.org/10.1002/1529-0131(199807)41:7<1266::AID-ART18>3.0.CO;2-Y (1998).
Notoya, K. et al. The induction of cell death in human osteoarthritis chondrocytes by nitric oxide is related to the production of prostaglandin E2 via the induction of cyclooxygenase-2. J. Immunol. 165, 3402–3410 (2000).
Scher, J. U., Pillinger, M. H. & Abramson, S. B. Nitric oxide synthases and osteoarthritis. Curr. Rheumatol. Rep. 9, 9–15 (2007).
Hellio le Graverand, M. P. et al. A 2-year randomised, double-blind, placebo-controlled, multicentre study of oral selective iNOS inhibitor, cindunistat (SD-6010), in patients with symptomatic osteoarthritis of the knee. Ann. Rheum. Dis. 72, 187–195 (2013).
Sutton, S. et al. The contribution of the synovium, synovial derived inflammatory cytokines and neuropeptides to the pathogenesis of osteoarthritis. Vet. J. 179, 10–24 (2009).
Meini, S. & Maggi, C. A. Knee osteoarthritis: a role for bradykinin? Inflamm. Res. 57, 351–361 (2008).
Song, I. H. et al. Contrast-enhanced ultrasound in monitoring the efficacy of a bradykinin receptor 2 antagonist in painful knee osteoarthritis compared with MRI. Ann. Rheum. Dis. 68, 75–83 (2009).
Dudek, M. et al. The chondrocyte clock gene Bmal1 controls cartilage homeostasis and integrity. J. Clin. Invest. 126, 365–376 (2016).
Kc, R. et al. Environmental disruption of circadian rhythm predisposes mice to osteoarthritis-like changes in knee joint. J. Cell. Physiol. 230, 2174–2183 (2015).
Gossan, N. et al. The circadian clock in murine chondrocytes regulates genes controlling key aspects of cartilage homeostasis. Arthritis Rheum. 65, 2334–2345 (2013).
Guo, B. et al. Catabolic cytokines disrupt the circadian clock and the expression of clock-controlled genes in cartilage via an NFκB-dependent pathway. Osteoarthritis Cartilage 23, 1981–1988 (2015).
Honda, K. K. et al. Different circadian expression of major matrix-related genes in various types of cartilage: modulation by light-dark conditions. J. Biochem. 154, 373–381 (2013).
Takarada, T. et al. Clock genes influence gene expression in growth plate and endochondral ossification in mice. J. Biol. Chem. 287, 36081–36095 (2012).
Gibbs, J. et al. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat. Med. 20, 919–926 (2014).
Nguyen, K. D. et al. Circadian gene Bmal1 regulates diurnal oscillations of Ly6Chi inflammatory monocytes. Science 341, 1483–1488 (2013).
Gibbs, J. E. et al. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc. Natl Acad. Sci. USA 109, 582–587 (2012).
Mobasheri, A. The future of osteoarthritis therapeutics: targeted pharmacological therapy. Curr. Rheumatol. Rep. 15, 364 (2013).
Mobasheri, A. The future of osteoarthritis therapeutics: emerging biological therapy. Curr. Rheumatol. Rep. 15, 385 (2013).
Hunter, D. J. Are there promising biologic therapies for osteoarthritis? Curr. Rheumatol. Rep. 10, 19–25 (2008).
Pelletier, J. P. et al. Disease-modifying effect of strontium ranelate in a subset of patients from the Phase III knee osteoarthritis study SEKOIA using quantitative MRI: reduction in bone marrow lesions protects against cartilage loss. Ann. Rheum. Dis. 74, 422–429 (2015).
Reginster, J. Y. et al. Efficacy and safety of strontium ranelate in the treatment of knee osteoarthritis: results of a double-blind, randomised placebo-controlled trial. Ann. Rheum. Dis. 72, 179–186 (2013).
Pelletier, J. P. et al. Strontium ranelate reduces the progression of experimental dog osteoarthritis by inhibiting the expression of key proteases in cartilage and of IL-1β in the synovium. Ann. Rheum. Dis. 72, 250–257 (2013).
Tat, S. K., Pelletier, J. P., Mineau, F., Caron, J. & Martel-Pelletier, J. Strontium ranelate inhibits key factors affecting bone remodeling in human osteoarthritic subchondral bone osteoblasts. Bone 49, 559–567 (2011).
Lafeber, F. P. & van Laar, J. M. Strontium ranelate: ready for clinical use as disease-modifying osteoarthritis drug? Ann. Rheum. Dis. 72, 157–161 (2013).
Felson, D. T. Developments in the clinical understanding of osteoarthritis. Arthritis Res. Ther. 11, 203 (2009).
Midwood, K. et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat. Med. 15, 774–780 (2009).
van Lent, P. L. et al. Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Rheum. 64, 1466–1476 (2012).
Schelbergen, R. F. et al. Alarmins S100A8/S100A9 aggravate osteophyte formation in experimental osteoarthritis and predict osteophyte progression in early human symptomatic osteoarthritis. Ann. Rheum. Dis. 75, 218–225 (2016).
Joosten, L. A., Helsen, M. M., van de Loo, F. A. & van den Berg, W. B. Anticytokine treatment of established type II collagen-induced arthritis in DBA/1 mice: a comparative study using anti-TNFα, anti-IL-1α/β and IL-1Ra. Arthritis Rheum. 58, S110–122 (2008).
Rudolphi, K., Gerwin, N., Verzijl, N., van der Kraan, P. & van den Berg, W. Pralnacasan, an inhibitor of interleukin-1β converting enzyme, reduces joint damage in two murine models of osteoarthritis. Osteoarthritis Cartilage 11, 738–746 (2003).
Zhang, Q., Lv, H., Chen, A., Liu, F. & Wu, X. Efficacy of infliximab in a rabbit model of osteoarthritis. Connect. Tissue Res. 53, 355–358 (2012).
Urech, D. M. et al. Anti-inflammatory and cartilage-protecting effects of an intra-articularly injected anti-TNFα single-chain Fv antibody (ESBA105) designed for local therapeutic use. Ann. Rheum. Dis. 69, 443–449 (2010).
Koewler, N. J. et al. Effects of a monoclonal antibody raised against nerve growth factor on skeletal pain and bone healing after fracture of the C57BL/6J mouse femur. J. Bone Miner. Res. 22, 1732–1742 (2007).
Moore, E. E. et al. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthritis Cartilage 13, 623–631 (2005).
Evans, C. H., Kraus, V. B. & Setton, L. A. Progress in intra-articular therapy. Nat. Rev. Rheumatol. 10, 11–22 (2014).
Ou, Y. et al. Selective COX-2 inhibitor ameliorates osteoarthritis by repressing apoptosis of chondrocyte. Med. Sci. Monit. 18, 247–252 (2012).
Bresalier, R. S. et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N. Engl. J. Med. 352, 1092–1102 (2005).
Drazen, J. M. COX-2 inhibitors — a lesson in unexpected problems. N. Engl. J. Med. 352, 1131–1132 (2005).
Nussmeier, N. A. et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N. Engl. J. Med. 352, 1081–1091 (2005).
Solomon, S. D. et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N. Engl. J. Med. 352, 1071–1080 (2005).
Riendeau, D. et al. Etoricoxib (MK-0663): preclinical profile and comparison with other agents that selectively inhibit cyclooxygenase-2. J. Pharmacol. Exp. Ther. 296, 558–566 (2001).
Song, G. G. et al. Relative efficacy and tolerability of etoricoxib, celecoxib, and naproxen in the treatment of osteoarthritis: a Bayesian network meta-analysis of randomized controlled trials based on patient withdrawal. Z. Rheumatol. 75, 508–516 (2016).
Croom, K. F. & Siddiqui, M. A. Etoricoxib: a review of its use in the symptomatic treatment of osteoarthritis, rheumatoid arthritis, ankylosing spondylitis and acute gouty arthritis. Drugs 69, 1513–1532 (2009).
Ratcliffe, A. et al. The in vivo effects of naproxen on canine experimental osteoarthritic articular cartilage: composition, metalloproteinase activities and metabolism. Agents Actions Suppl. 39, 207–211 (1993).
Svensson, O., Malmenas, M., Fajutrao, L., Roos, E. M. & Lohmander, L. S. Greater reduction of knee than hip pain in osteoarthritis treated with naproxen, as evaluated by WOMAC and SF-36. Ann. Rheum. Dis. 65, 781–784 (2006).
Coxib and traditional NSAID Trialists' (CNT) Collaboration et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 382, 769–779 (2013).
Masferrer, J. L. et al. Pharmacology of PF-4191834, a novel, selective non-redox 5-lipoxygenase inhibitor effective in inflammation and pain. J. Pharmacol. Exp. Ther. 334, 294–301 (2010).
Jovanovic, D. V. et al. In vivo dual inhibition of cyclooxygenase and lipoxygenase by ML-3000 reduces the progression of experimental osteoarthritis: suppression of collagenase 1 and interleukin-1β synthesis. Arthritis Rheum. 44, 2320–2330 (2001).
Raynauld, J. P. et al. Protective effects of licofelone, a 5-lipoxygenase and cyclo-oxygenase inhibitor, versus naproxen on cartilage loss in knee osteoarthritis: a first multicentre clinical trial using quantitative MRI. Ann. Rheum. Dis. 68, 938–947 (2009).
Bitto, A. et al. Flavocoxid, a nutraceutical approach to blunt inflammatory conditions. Mediators Inflamm. 2014, 790851 (2014).
Levy, R. M. et al. Efficacy and safety of flavocoxid, a novel therapeutic, compared with naproxen: a randomized multicenter controlled trial in subjects with osteoarthritis of the knee. Adv. Ther. 27, 731–742 (2010).
Chalasani, N. et al. Acute liver injury due to flavocoxid (Limbrel), a medical food for osteoarthritis: a case series. Ann. Intern. Med. 156, 857–860 (2012).
Stefanovic-Racic, M. et al. N-Monomethyl arginine, an inhibitor of nitric oxide synthase, suppresses the development of adjuvant arthritis in rats. Arthritis Rheum. 37, 1062–1069 (1994).
McCartney-Francis, N. et al. Suppression of arthritis by an inhibitor of nitric oxide synthase. J. Exp. Med. 178, 749–754 (1993).
Miyasaka, N. & Hirata, Y. Nitric oxide and inflammatory arthritides. Life Sci. 61, 2073–2081 (1997).
More, A. S. et al. Effect of iNOS inhibitor S-methylisothiourea in monosodium iodoacetate-induced osteoathritic pain: implication for osteoarthritis therapy. Pharmacol. Biochem. Behav. 103, 764–772 (2013).
Brewster, M., Lewis, E. J., Wilson, K. L., Greenham, A. K. & Bottomley, K. M. Ro 32–3555, an orally active collagenase selective inhibitor, prevents structural damage in the STR/ORT mouse model of osteoarthritis. Arthritis Rheum. 41, 1639–1644 (1998).
Close, D. R. Matrix metalloproteinase inhibitors in rheumatic diseases. Ann. Rheum. Dis. 60 (Suppl. 3), 62–67 (2001).
Fujisawa, T. et al. Highly water-soluble matrix metalloproteinases inhibitors and their effects in a rat adjuvant-induced arthritis model. Bioorg. Med. Chem. 10, 2569–2581 (2002).
Janusz, M. J. et al. Moderation of iodoacetate-induced experimental osteoarthritis in rats by matrix metalloproteinase inhibitors. Osteoarthritis Cartilage 9, 751–760 (2001).
Lewis, E. J. et al. Ro 32–3555, an orally active collagenase inhibitor, prevents cartilage breakdown in vitro and in vivo. Br. J. Pharmacol. 121, 540–546 (1997).
Acknowledgements
The authors' research is supported by the following awards (to W.H.R.): US Department of Veterans Affairs Merit Review Awards I01BX002345, I01RX000934 and I01RX000588; NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), National Institute of Allergy and Infectious Diseases (NIAID) and the Foundation for the NIH Accelerating Medicines Partnership Program UH2 AR067681; and the Northern California Chapter of the Arthritis Foundation (NCCAF) Center of Excellence.
Author information
Authors and Affiliations
Contributions
W.H.R. and R.M. wrote the article. All authors researched the data for the article, contributed substantially to discussions of its content, and participated in review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J.S. is an employee of AbbVie. All other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Robinson, W., Lepus, C., Wang, Q. et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol 12, 580–592 (2016). https://doi.org/10.1038/nrrheum.2016.136
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2016.136
This article is cited by
-
Saikosaponin D alleviates inflammatory response of osteoarthritis and mediates autophagy via elevating microRNA-199-3p to target transcription Factor-4
Journal of Orthopaedic Surgery and Research (2024)
-
Glaucocalyxin A delays the progression of OA by inhibiting NF-κB and MAPK signaling pathways
Journal of Orthopaedic Surgery and Research (2024)
-
ADAM8 silencing suppresses the migration and invasion of fibroblast-like synoviocytes via FSCN1/MAPK cascade in osteoarthritis
Arthritis Research & Therapy (2024)
-
Metabolic syndrome increases osteoarthritis risk: findings from the UK Biobank prospective cohort study
BMC Public Health (2024)
-
Specific-cytokine associations with outcomes in knee osteoarthritis subgroups: breaking down disease heterogeneity with phenotyping
Arthritis Research & Therapy (2024)