Key Points
-
Vessels are more than merely conduits for blood, nutrients, gas exchange and waste disposal
-
The dialogue between developing and mature vessels and their resident tissues determines organ form, function, specialization, vulnerability and capacity for repair
-
Vessels of the same size in different organs are not the same, reflecting specialized functions
-
Vessels are immunologically competent structures
-
As with other tissues, growth, development and ageing of vessels are associated with adaptations (and maladaptations) that modify their function and vulnerabilities
-
The unique features that define vascular diversity provide extraordinary opportunities to explore mechanisms responsible for unique disease patterns in different forms of vasculitis
Abstract
The vasculitides are a large group of heterogeneous diseases for which it has been assumed that pathogenesis is largely autoimmune. As clinicians, we distinguish one form of vasculitis from another on the basis of observed patterns of organ injury, the size of the vessels affected and histopathological findings. The terms 'small-vessel', 'medium-vessel' and 'large-vessel' vasculitis are useful clinical descriptors, but fail to inform us about why vessels of a certain calibre are favoured by one disease and not another. Classification based on vessel size also fails to consider that vessels of a specific calibre are not equally prone to injury. Distinct vulnerabilities undoubtedly relate to the fact that same-size vessels in different tissues may not be identical conduits. In fact, vessels become specialized, from the earliest stages of embryonic development, to suit the needs of different anatomical locations. Vessels of the same calibre in different locations and organs are as different as the organ parenchymal cells through which they travel. The dialogue between developing vessels and the tissues they perfuse is designed to meet special local needs. Added to the story of vascular diversity and vulnerability are changes that occur during growth, development and ageing. An improved understanding of the unique territorial vulnerabilities of vessels could form the basis of new hypotheses for the aetiopathogenesis of the vasculitides. This Review considers how certain antigens, including infectious agents, might become disease-relevant and how vascular diversity could influence disease phenotypes and the spectrum of vascular inflammatory diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Rocha, S. F. & Adams, R. H. Molecular differentiation and specialization of vascular beds. Angiogenesis 12, 139–147 (2009).
Cleaver, O. & Melton, D. A. Endothelial signaling during development. Nat. Med. 9, 661–668 (2003).
Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 438, 932–936 (2005).
Swift, M. R. & Weinstein, B. M. Arterial-venous specification during development. Circ. Res. 104, 576–588 (2009).
Larrivée, B., Freitas, C., Suchting, S., Brunet, I. & Eichmann, A. Guidance of vascular development: lessons from the nervous system. Circ. Res. 104, 428–441 (2009).
Ribatti, D., Nico, B. & Crivellato, E. Morphological and molecular aspects of physiological vascular morphogenesis. Angiogenesis 12, 101–111 (2009).
Davis, G. E. & Senger, D. R. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ. Res. 97, 1093–1107 (2005).
Hallmann, R. et al. Expression and function of laminins in the embryonic and mature vasculature. Physiology Rev. 85, 979–1000 (2005).
Stan, R. V. Endothelial stomatal and fenestral diaphragms in normal vessels and angiogenesis. J. Cell. Mol. Med. 11, 621–643 (2007).
Pries, A. R. & Kuebler, W. M. Normal endothelium. Handbook Exptl Pharmacol. 176, 1–40 (2006).
Sá-Pereira, I., Brites, D. & Brito, M. A. Neurovascular unit: a focus on pericytes. Mol. Neurobiol. 45, 327–347 (2012).
Molema, G. & Aird, W. C. Vascular heterogeneity in the kidney. Semin. Nephrol. 32, 145–155 (2012).
Okuyama, K., Yaginuma, G., Takahashi, T., Sasaki, H. & Mori, S. The development of vasa vasorum of the human aorta in various conditions. A morphometric study. Arch. Pathol. Lab. Med. 112, 721–725 (1988).
Margolin, D. A. et al. Differential monocytic cell adherence to specific anatomic regions of the canine aorta. J. Vasc. Res. 32, 266–274 (1995).
Topouzis, S. & Majesky, M. W. Smooth muscle lineage diversity in the chick embryo: two types of aortic smooth muscle cell differ in growth and receptor-mediated transcriptional responses to transforming growth factor-β. Dev. Biol. 178, 430–445 (1996).
Majesky, M. W. Developmental basis of vascular smooth muscle diversity. Arterioscler. Thromb. Vasc. Biol. 27, 1248–1258 (2007).
Pryshchep, O., Ma-Krupa, W., Younge, B. R., Goronzy, J. J. & Weyand, C. M. Vessel-specific Toll-like receptor profiles in human medium and large arteries. Circulation 118, 1276–1284 (2008).
Foster, T. J., Geoghegan, J. A., Ganesh, V. K. & Höök, M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 12, 49–62 (2014).
Vengadesan, K. & Narayana, V. L. Structural biology of Gram-positive bacterial adhesins. Protein Sci. 20, 759–772 (2011).
Belouzard, S., Millet, J. K., Licitra, B. N. & Whittaker, G. R. Mechanisms of Coronavirus cell entry mediated by the viral spike protein. Viruses 4, 1011–1033 (2012).
Cuperus, T., Coorens M, van Dijk, A. & Haagsman, H. P. Avian host defense peptides. Dev. Comp. Immunol. 41, 352–369 (2013).
Zhao, L. & Lu, W. Defensins in innate immunity. Curr. Opin. Hematol. 21, 37–42 (2014).
Guillevin, L. Infections in vasculitis. Best Pract. Res. Clin. Rheumatol. 27, 19–31 (2013).
Calabrese, L. H. Infection with the human immunodeficiency virus type 1 and vascular inflammatory disease. Clin. Exp. Rheumatol. 22 (Suppl. 36), S87–S93 (2004).
Weck, K. E. et al. Murine γ-herpesvirus 68 causes severe large-vessel arteritis in mice lacking interferon-γ responsiveness: a new model for virus-induced vascular disease. Nat. Med. 3, 1346–1353 (1997).
Presti, R. M., Pollock, J. L., Dal Canto, A. J., O'Guin, A. K. & Virgin, H. W. Interferon γ regulates acute and latent murine cytomegalovirus infection and chronic disease of the great vessels. J. Exp. Med. 188, 577–588 (1998).
Dal Canto, A. J., Swanson, P. E., O'Guin, A. K., Speck, S. H. & Virgin, H. W. IFN-γ action in the media of the great elastic arteries, a novel immunoprivileged site. J. Clin. Invest. 107, R15–R22 (2001).
Agnello, V., Chung, R. T. & Kaplan, L. M. A role for hepatitis C virus infection in type II cryoglobulinemia. N. Engl. J. Med. 327, 1490–1495 (1992).
Antonelli, A. et al. Serum concentrations of interleukin 1β, CXCL10, and interferon-γ in mixed cryoglobulinemia associated with hepatitis C infection. J. Rheumatol. 37, 91–97 (2013).
Sansonno, D. & Dammacco, F. Hepatitis C virus, cryoglobulinaemia, and vasculitis: immune complex relations. Lancet Infect. Dis. 5, 227–236 (2005).
Fabrizi, F. et al. Hepatitis C virus infection, mixed cryoglobulinemia, and kidney disease. Am. J. Kidney Dis. 61, 623–637 (2013).
Cacoub, P., Terrier, B. & Saadoun, D. Hepatitis C virus-induced vasculitis: therapeutic options. Ann. Rheum. Dis. 73, 24–30 (2014).
Ferri, C. et al. HCV-related autoimmune and neoplastic disorders: the HCV syndrome. Dig. Liver Dis. 39 (Suppl. 1), S13–S21 (2007).
Saadoun, D. et al. Hepatitis C virus-associated polyarteritis nodosa. Arthritis Care Res. (Hoboken) 63, 427–435 (2011).
Zeisel, M. B., Felmlee, D. J. & Baumert, T. F. Hepatitis C viral entry. Curr. Topics Microbiol. Immunol. 369, 86–112 (2013).
Catanese, M. T. et al. Different requirements for scavenger receptor class B type I in hepatitis C virus cell-free versus cell-to-cell transmission. J. Virol. 87, 8282–8293 (2013).
Ferri, C., Zignego, A. L. & Pileri, S. A. Cryoglobulins. J. Clin. Pathol. 55, 4–13 (2002).
Alpers, C. E. & Smith, K. D. Cryoglobulinemia and renal disease. Curr. Opin. Nephrol. Hypertens. 17, 243–249 (2008).
Gorevic, P. D. Rheumatoid factor, complement, and mixed cryoglobulinemia. Clin. Dev. Immunol. 2012, 439018 (2012).
Sansonno, D. et al. Increased serum levels of the chemokine CXCL13 and up-regulation of its gene expression are distinctive features of HCV-related cryoglobulinemia and correlate with active cutaneous vasculitis. Blood 112, 1620–1627 (2008).
Sansonno, D. B. et al. Role of the receptor for the globular domain of C1q protein in the pathogenesis of hepatitis C virus-related cryoglobulin vascular damage. J. Immunol. 183, 6013–6020 (2009).
Dammacco, F. & Sansonno, D. Therapy for hepatitis C virus-related cryoglobulinemic vasculitis. N. Engl. J. Med. 369, 1035–1045 (2013).
Fabrizi, F. et al. Biological dynamics of hepatitis B virus load in dialysis population. Am. J. Kidney Dis. 41, 1278–1285 (2003).
Fornasieri, A. & D'Amico, G. Type II mixed cryoglobulinaemia, hepatitis C virus infection, and glomerulonephritis. Nephrol. Dial. Transplant. 11 (Suppl. 4), 25–30 (1996).
Cacoub, P. et al. Anti-endothelial cell auto-antibodies in hepatitis C virus mixed cryoglobulinemia. J. Hepatol. 31, 598–603 (1999).
Barsoum, R. S. Hepatitis C virus: from entry to renal injury—facts and potentials. Nephrol. Dial. Transplant. 22, 1840–1848 (2007).
Sansonno, D. et al. Hepatitis C virus RNA and core protein in kidney glomerular and tubular structures isolated with laser capture microdissection. Clin. Exp. Immunol. 140, 498–506 (2005).
Saadoun, D. et al. Involvement of chemokines and type 1 cytokines in the pathogenesis of hepatitis C virus-associated mixed cryoglobulinemia vasculitis neuropathy. Arthritis Rheum. 52, 2917–2925 (2005).
Wornle, M. et al. Novel role of Toll-like receptor 3 in hepatitis C-associated glomerulonephritis. Am. J. Pathol. 168, 370–385 (2006).
Authier, F. J. et al. Detection of genomic viral RNA in nerve and muscle of patients with HCV neuropathy. Neurology 60, 808–812 (2003).
Fletcher, N. F. et al. Hepatitis C virus infects the endothelial cells of the blood–brain barrier. Gastroenterology 142, 634–643 (2012).
Forton, D. M., Karayiannis, P., Mahmud, N., Taylor-Robinson, S. D. & Thomas, H. C. Identification of unique hepatitis C virus quasispecies in the central nervous system and comparative analysis of internal translational efficiency of brain, liver, and serum variants. J. Virol. 78, 5170–5183 (2004).
Sabahi, A. Hepatitis C virus entry: the early steps in the viral replication cycle. Virol. J. 6, 117 (2009).
Feray, C. Is HCV infection a neurologic disorder? Gastroenterology 142, 428–431 (2012).
Burlone, M. E. & Budkowska, A. Hepatitis C virus cell entry: role of lipoproteins and cellular receptors. J. Gen. Virol. 90, 1055–1070 (2009).
Wilkinson, J., Radkowski, M. & Laskus, T. Hepatitis C virus neuroinvasion: identification of infected cells. J. Virol. 83, 1312–1319 (2009).
Fletcher, N. F. et al. Activated macrophages promote hepatitis C virus entry in a tumor necrosis factor-dependent manner. Hepatology 59, 1320–1330 (2014).
Jia, X. Y., Cui, Z., Yang, R., Hu, S. Y. & Zhao, M. H. Antibodies against linear epitopes on the Goodpasture autoantigen and kidney injury. Clin. J. Am. Soc. Nephrol. 7, 926–933 (2012).
Vanacore, R., Pedchenko, V., Bhave, G. & Hudson, B. G. Sulphilimine cross-links in Goodpasture's disease. Clin. Exp. Immunol. 164, 4–6 (2011).
Peto, P. & Salama, A. D. Update on antiglomerular basement membrane disease. Curr. Opin. Rheumatol. 23, 32–37 (2011).
Ooi, J. D. et al. The HLA-DRB1*15:01-restricted Goodpasture's T cell epitope induces GN. J. Am. Soc. Nephrol. 24, 419–431 (2013).
Doyle, H. A. & Mamula, M. J. Autoantigenesis: the evolution of protein modifications in autoimmune disease. Curr. Opin. Immunol. 24, 112–118 (2012).
Hernández-Rodriguez, J. et al. Vasculitis involving the breast: a clinical and histological analysis of 34 patients. Medicine (Baltimore) 87, 61–69 (2008).
Hernández-Rodríguez, J., Tan, C. D., Rodríguez, R. E. & Hoffman, G. S. Gynecologic vasculitis: an analysis of 163 patients. Medicine (Baltimore) 88, 169–181 (2009).
Hernández-Rodríguez, J. & Hoffman, G. S. Updating single-organ vasculitis. Curr. Opin. Rheumatol. 24, 38–45 (2012).
Jennette, J. C. et al. Revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum. 65, 1–11 (2013).
Falk, R. J. & Hoffman, G. S. Controversies in small vessel vasculitis—comparing rheumatology and nephrology views. Curr. Opin. Rheumatol. 19, 1–9 (2007).
Sablé-Fourtassou, R. et al. Antineutrophil cytoplasm antibodies and the Churg–Strauss syndrome. Ann. Intern. Med. 143, 632–638 (2005).
Sinico, R. A. et al. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in Churg–Strauss syndrome. Arthritis Rheum. 52, 2926–2935 (2005).
Ribatti, D., Nico, B., Vacca, A., Roncali, L. & Dammacco, F. Endothelial cell heterogeneity and organ specificity. J. Hematother. Stem Cell Res. 11, 81–90 (2002).
Mohan, S., Liao, Y., Kim, J., Goronzy, J. & Weyand, C. Giant cell arteritis: immune and vascular aging as disease risk factors. Arthritis Res. Ther. 13, 231 (2011).
Maksimowicz-McKinnon, K., Clark, T. M. & Hoffman, G. S. Takayasu arteritis and giant cell arteritis: a spectrum within the same disease? Medicine (Baltimore) 88, 221–226 (2009).
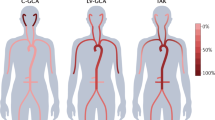
Grayson, P. C. et al. Distribution of arterial lesions in Takayasu's arteritis and giant cell arteritis. Ann. Rheum. Dis. 71, 1329–1334 (2012).
Zivkovic, S. A., Clemens, P. R. & Lacomis, D. Characteristics of late-onset myasthenia gravis. J. Neurol. 259, 2167–2171 (2012).
Aarli, J. A. Myasthenia gravis in the elderly: is it different? Ann. N. Y. Acad. Sci. 1132, 238–243 (2008).
Acknowledgements
G.S.H. has received partial research support from the Harold C. Schott Foundation and the Konigsberg Family Fund for Vasculitis Research. L.H.C. is in part supported by the R. J. Fasenmyer Foundation.
Author information
Authors and Affiliations
Contributions
G.S.H. conceived the article's content, researched data for the article, wrote the first draft and reviewed/edited the manuscript before submission. L.H.C. researched data for and wrote the section on viral-associated vasculitis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Hoffman, G., Calabrese, L. Vasculitis: determinants of disease patterns. Nat Rev Rheumatol 10, 454–462 (2014). https://doi.org/10.1038/nrrheum.2014.89
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2014.89
This article is cited by
-
Central and Peripheral Nervous System Complications of Vasculitis Syndromes from Pathology to Bedside: Part 2—Peripheral Nervous System
Current Neurology and Neuroscience Reports (2023)
-
The impact of the COVID-19 pandemic on patients with systemic vasculitis: a single-centre retrospective study
Rheumatology International (2023)
-
Aortic involvement in relapsing polychondritis: case-based review
Rheumatology International (2021)
-
Classification of ANCA-associated vasculitis: differences based on ANCA specificity and clinicopathologic phenotype
Rheumatology International (2021)
-
Mechanism and biomarkers in aortitis––a review
Journal of Molecular Medicine (2020)