Key Points
-
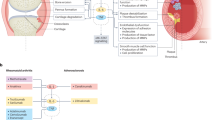
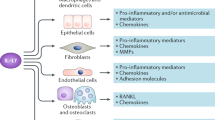
Eicosanoid pathways have a major role in the pathogenesis of rheumatic diseases, including rheumatoid arthritis, osteoarthritis, idiopathic inflammatory myositis, systemic lupus erythematosus and gouty arthritis
-
Despite potent suppression of specific inflammatory mechanisms by a wide range of antirheumatic drugs, the cyclooxygenase and 5-lipoxygenase pathways remain overexpressed and can contribute to subclinical inflammation and relapse of rheumatic disease
-
Several enzymes of the arachidonic acid cascade (part of the eicosanoid pathway) and eicosanoid receptors are well-recognized targets for anti-inflammatory drugs that can reduce symptoms of inflammation in rheumatic diseases
-
The combination of microsomal prostaglandin E synthase 1 inhibition with current antirheumatic treatments or with leukotriene inhibitors might improve the effect of anti-inflammatory agents in certain disorders
Abstract
An unmet clinical need exists for early treatment of rheumatic diseases and improved treatment strategies that can better maintain remission with reduced ongoing subclinical inflammation and bone destruction. Eicosanoids form one of the most complex networks in the body controlling many physiological and pathophysiological processes, including inflammation, autoimmunity and cancer. Persisting eicosanoid pathways are thought to be involved in the development of rheumatic diseases, and targeting this pathway might enable improved treatment strategies. Several enzymes of the arachidonic acid cascade as well as eicosanoid receptors (all part of the eicosanoid pathway) are today well-recognized targets for anti-inflammatory drugs that can reduce symptoms of inflammation in rheumatic diseases. In this Review, we outline the evidence supporting pivotal roles of eicosanoid signalling in the pathogenesis of rheumatic diseases and discuss findings from studies in animals and humans. We focus first on rheumatoid arthritis and discuss the upregulation of the cyclooxygenase and lipoxygenase pathways as most data are available in this condition. Research into the roles of eicosanoids in other rheumatic diseases (osteoarthritis, idiopathic inflammatory myopathies, systemic lupus erythematosus and gout) is also progressing rapidly and is discussed. Finally, we summarize the prospects of targeting eicosanoid pathways as anti-inflammatory treatment strategies for patients with rheumatic diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Funk, C. D. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294, 1871–1875 (2001).
Ricciotti, E. & FitzGerald, G. A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 31, 986–1000 (2011).
Stables, M. J. & Gilroy, D. W. Old and new generation lipid mediators in acute inflammation and resolution. Prog. Lipid Res. 50, 35–51 (2011).
Korotkova, M. et al. Effects of antirheumatic treatments on the prostaglandin E2 biosynthetic pathway. Arthritis Rheum. 52, 3439–3447 (2005).
Korotkova, M. et al. Effects of immunosuppressive treatment on microsomal prostaglandin E synthase 1 and cyclooxygenases expression in muscle tissue of patients with polymyositis or dermatomyositis. Ann. Rheum. Dis. 67, 1596–1602 (2008).
Gheorghe, K. R. et al. Prostaglandin E2 synthesizing enzymes in rheumatoid arthritis B cells and the effects of B cell depleting therapy on enzyme expression. PLoS ONE 6, e16378 (2011).
Gheorghe, K. R. et al. Limited effect of anti-rheumatic treatment on 15-prostaglandin dehydrogenase in rheumatoid arthritis synovial tissue. Arthritis Res. Ther. 14, R121 (2012).
Grosser, T., Yu, Y. & Fitzgerald, G. A. Emotion recollected in tranquility: lessons learned from the COX-2 saga. Annu. Rev. Med. 61, 17–33 (2010).
Smith, W. L., Urade, Y. & Jakobsson, P. J. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem. Rev. 111, 5821–5865 (2011).
Samuelsson, B., Morgenstern, R. & Jakobsson, P. J. Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol. Rev. 59, 207–224 (2007).
Korotkova, M. & Jakobsson, P. J. Characterization of microsomal prostaglandin E synthase 1 Inhibitors. Basic Clin. Pharmacol. Toxicol. 114, 64–69 (2014).
Korotkova, M. & Jakobsson, P. J. Microsomal prostaglandin E synthase-1 in rheumatic diseases. Front. Pharmacol. 1, 1–8 (2010).
Scott, J. P. & Peters-Golden, M. Antileukotriene agents for the treatment of lung disease. Am. J. Res. Crit. Care Med. 188, 538–544 (2013).
Bombardieri, S. et al. The synovial prostaglandin system in chronic inflammatory arthritis: differential effects of steroidal and nonsteroidal anti-inflammatory drugs. Br. J. Pharmacol. 73, 893–901 (1981).
Raichel, L. et al. Reduction of cPLA2α overexpression: an efficient anti-inflammatory therapy for collagen-induced arthritis. Eur. J. Immunol. 38, 2905–2915 (2008).
Tai, N. et al. Cytosolic phospholipase A2 alpha inhibitor, pyrroxyphene, displays anti-arthritic and anti-bone destructive action in a murine arthritis model. Inflamm. Res. 59, 53–62 (2010).
Hegen, M. et al. Cytosolic phospholipase A2α-deficient mice are resistant to collagen-induced arthritis. J. Exp. Med. 197, 1297–1302 (2003).
Anderson, G. D. et al. Selective inhibition of cyclooxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis. J. Clin. Invest. 97, 2672–2679 (1996).
Gebhard, H. H. et al. The effects of celecoxib on inflammation and synovial microcirculation in murine antigen-induced arthritis. Clin. Exp. Rheum. 23, 63–70 (2005).
Myers, L. K. et al. The genetic ablation of cyclooxygenase 2 prevents the development of autoimmune arthritis. Arthritis Rheum. 43, 2687–2693 (2000).
Mancini, J. A. et al. Cloning, expression, and up-regulation of inducible rat prostaglandin e synthase during lipopolysaccharide-induced pyresis and adjuvant-induced arthritis. J. Biol. Chem. 276, 4469–4475 (2001).
Engblom, D. et al. Induction of microsomal prostaglandin E synthase in the rat brain endothelium and parenchyma in adjuvant-induced arthritis. J. Comp. Neurol. 452, 205–214 (2002).
Trebino, C. E. et al. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc. Natl Acad. Sci. USA 100, 9044–9049 (2003).
Kojima, F. et al. Defective generation of a humoral immune response is associated with a reduced incidence and severity of collagen-induced arthritis in microsomal prostaglandin E synthase-1 null mice. J. Immunol. 180, 8361–8368 (2008).
Tsubouchi, Y. et al. Feedback control of the arachidonate cascade in rheumatoid synoviocytes by 15-deoxy-Δ12,14-prostaglandin J2. Biochem. Biophys. Res. Commun. 283, 750–755 (2001).
Li, X. et al. Expression and regulation of microsomal prostaglandin E synthase-1 in human osteoarthritic cartilage and chondrocytes. J. Rheumatol. 32, 887–895 (2005).
Kawahito, Y. et al. 15-deoxy-Δ12,14-PGJ2 induces synoviocyte apoptosis and suppresses adjuvant-induced arthritis in rats. J. Clin. Invest. 106, 189–197 (2000).
Cuzzocrea, S. et al. The cyclopentenone prostaglandin 15-deoxy-Δ12,14-prostaglandin J2 attenuates the development of acute and chronic inflammation. Mol. Pharmacol. 61, 997–1007 (2002).
Bell-Parikh, L. C. et al. Biosynthesis of 15-deoxy-Δ12,14-PGJ2 and the ligation of PPARγ. J. Clin. Invest. 112, 945–955 (2003).
Maicas, N., Ibanez, L., Alcaraz, M. J., Ubeda, A. & Ferrandiz, M. L. Prostaglandin D2 regulates joint inflammation and destruction in murine collagen-induced arthritis. Arthritis Rheum. 64, 130–140 (2012).
Pulichino, A. M. et al. Prostacyclin antagonism reduces pain and inflammation in rodent models of hyperalgesia and chronic arthritis. J. Pharmacol. Exp. Ther. 319, 1043–1050 (2006).
Honda, T., Segi-Nishida, E., Miyachi, Y. & Narumiya, S. Prostacyclin-IP signaling and prostaglandin E2-EP2/EP4 signaling both mediate joint inflammation in mouse collagen-induced arthritis. J. Exp. Med. 203, 325–335 (2006).
Hishinuma, T. et al. Analysis of urinary prostacyclin and thromboxane/prostacyclin ratio in patients with rheumatoid arthritis using gas chromatography/selected ion monitoring. Prostaglandins Leukot. Essent. Fatty Acids 65, 85–90 (2001).
Nakamura, H. et al. Difference in urinary 11-dehydro TXB2 and LTE4 excretion in patients with rheumatoid arthritis. Prostaglandins Leukot. Essent. Fatty Acids 65, 301–306 (2001).
Egg, D. Concentrations of prostaglandins D2, E2, F2α, 6-keto-F1α and thromboxane B2 in synovial fluid from patients with inflammatory joint disorders and osteoarthritis. Z. Rheumol. 43, 89–96 (1984).
Leistad, L., Feuerherm, A. J., Ostensen, M., Faxvaag, A. & Johansen, B. Presence of secretory group IIa and V phospholipase A2 and cytosolic group IValpha phospholipase A2 in chondrocytes from patients with rheumatoid arthritis. Clin. Chem. Lab. Med. 42, 602–610 (2004).
Hulkower, K. I. et al. Interleukin-1β induces cytosolic phospholipase A2 and prostaglandin H synthase in rheumatoid synovial fibroblasts. Evidence for their roles in the production of prostaglandin E2. Arthritis Rheum. 37, 653–661 (1994).
Crofford, L. J. COX-1 and COX-2 tissue expression: implications and predictions. J. Rheumatol. Suppl. 49, 15–19 (1997).
Siegle, I. et al. Expression of cyclooxygenase 1 and cyclooxygenase 2 in human synovial tissue: differential elevation of cyclooxygenase 2 in inflammatory joint diseases. Arthritis Rheum. 41, 122–129 (1998).
Westman, M. et al. Expression of microsomal prostaglandin E synthase 1 in rheumatoid arthritis synovium. Arthritis Rheum. 50, 1774–1780 (2004).
Murakami, M. et al. Cellular prostaglandin E2 production by membrane-bound prostaglandin E synthase-2 via both cyclooxygenases-1 and -2. J. Biol. Chem. 278, 37937–37947 (2003).
Murakami, M. et al. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J. Biol. Chem. 275, 32783–32792 (2000).
Lazarus, M. et al. Biochemical characterization of mouse microsomal prostaglandin E synthase-1 and its colocalization with cyclooxygenase-2 in peritoneal macrophages. Arch. Biochem. Biophys. 397, 336–341 (2002).
Thoren, S. & Jakobsson, P. J. Coordinate up- and down-regulation of glutathione-dependent prostaglandin E synthase and cyclooxygenase-2 in A549 cells. Inhibition by NS-398 and leukotriene C4. Eur. J. Biochem. 267, 6428–6434 (2000).
Dieter, P. et al. Functional coupling of cyclooxygenase 1 and 2 to discrete prostanoid synthases in liver macrophages. Biochem. Biophys. Res. Commun. 276, 488–492 (2000).
Stichtenoth, D. O. et al. Microsomal prostaglandin E synthase is regulated by proinflammatory cytokines and glucocorticoids in primary rheumatoid synovial cells. J. Immunol. 167, 469–474 (2001).
Kojima, F. et al. Prostaglandin E2 is an enhancer of interleukin-1β-induced expression of membrane-associated prostaglandin E synthase in rheumatoid synovial fibroblasts. Arthritis Rheum. 48, 2819–2828 (2003).
Nah, S. S. et al. Epidermal growth factor increases prostaglandin E2 production via ERK1/2 MAPK and NF-κB pathway in fibroblast like synoviocytes from patients with rheumatoid arthritis. Rheumatol. Int. 30, 443–449 (2010).
Kusunoki, N. et al. Adiponectin stimulates prostaglandin E2 production in rheumatoid synovial fibroblasts. Arthritis Rheum. 62, 1641–1649 (2010).
Jungel, A. et al. Microparticles stimulate the synthesis of prostaglandin E2 via induction of cyclooxygenase 2 and microsomal prostaglandin E synthase 1. Arthritis Rheum. 56, 3564–3574 (2007).
Tong, M., Ding, Y. & Tai, H. H. Reciprocal regulation of cyclooxygenase-2 and 15-hydroxyprostaglandin dehydrogenase expression in A549 human lung adenocarcinoma cells. Carcinogenesis 27, 2170–2179 (2006).
Newton, R., Seybold, J., Kuitert, L. M., Bergmann, M. & Barnes, P. J. Repression of cyclooxygenase-2 and prostaglandin E2 release by dexamethasone occurs by transcriptional and post-transcriptional mechanisms involving loss of polyadenylated mRNA. J. Biol. Chem. 273, 32312–32321 (1998).
Dolhain, R. J. et al. Methotrexate reduces inflammatory cell numbers, expression of monokines and of adhesion molecules in synovial tissue of patients with rheumatoid arthritis. Br. J. Rheumatol. 37, 502–508 (1998).
Mello, S. B., Barros, D. M., Silva, A. S., Laurindo, I. M. & Novaes, G. S. Methotrexate as a preferential cyclooxygenase 2 inhibitor in whole blood of patients with rheumatoid arthritis. Rheumatology (Oxford) 39, 533–536 (2000).
Vergne, P. et al. Methotrexate and cyclooxygenase metabolism in cultured human rheumatoid synoviocytes. J. Rheumatol. 25, 433–440 (1998).
Seitz, M., Loetscher, P., Dewald, B., Towbin, H. & Baggiolini, M. In vitro modulation of cytokine, cytokine inhibitor, and prostaglandin E release from blood mononuclear cells and synovial fibroblasts by antirheumatic drugs. J. Rheumatol. 24, 1471–1476 (1997).
Tracey, D., Klareskog, L., Sasso, E. H., Salfeld, J. G. & Tak, P. P. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol. Ther. 117, 244–279 (2008).
Catrina, A. I. et al. Evidence that anti-tumor necrosis factor therapy with both etanercept and infliximab induces apoptosis in macrophages, but not lymphocytes, in rheumatoid arthritis joints: extended report. Arthritis Rheum. 52, 61–72 (2005).
Thurlings, R. M. et al. Synovial tissue response to rituximab: mechanism of action and identification of biomarkers of response. Ann. Rheum. Dis. 67, 917–925 (2008).
Klickstein, L. B., Shapleigh, C. & Goetzl, E. J. Lipoxygenation of arachidonic acid as a source of polymorphonuclear leukocyte chemotactic factors in synovial fluid and tissue in rheumatoid arthritis and spondyloarthritis. J. Clin. Invest. 66, 1166–1170 (1980).
Davidson, E. M., Rae, S. A. & Smith, M. J. Leukotriene B4, a mediator of inflammation present in synovial fluid in rheumatoid arthritis. Ann. Rheum. Dis. 42, 677–679 (1983).
Elmgreen, J., Nielsen, O. H. & Ahnfelt-Ronne, I. Enhanced capacity for release of leucotriene B4 by neutrophils in rheumatoid arthritis. Ann. Rheum. Dis. 46, 501–505 (1987).
Ahmadzadeh, N., Shingu, M., Nobunaga, M. & Tawara, T. Relationship between leukotriene B4 and immunological parameters in rheumatoid synovial fluids. Inflammation 15, 497–503 (1991).
Koshihara, Y., Isono, T., Oda, H., Karube, S. & Hayashi, Y. Measurement of sulfidopeptide leukotrienes and their metabolism in human synovial fluid of patients with rheumatoid arthritis. Prostaglandins Leukot. Essent. Fatty Acids 32, 113–119 (1988).
Xu, S., Lu, H., Lin, J., Chen, Z. & Jiang, D. Regulation of TNFα and IL1β in rheumatoid arthritis synovial fibroblasts by leukotriene B4. Rheumatol. Int. 30, 1183–1189 (2010).
Chen, M. et al. Joint tissues amplify inflammation and alter their invasive behavior via leukotriene B4 in experimental inflammatory arthritis. J. Immunol. 185, 5503–5511 (2010).
Chen, H. et al. Effects of leukotriene B4 and prostaglandin E2 on the differentiation of murine Foxp3+ T regulatory cells and TH17 cells. Prostaglandins Leukot. Essent. Fatty Acids 80, 195–200 (2009).
Garcia, C. et al. Leukotriene B4 stimulates osteoclastic bone resorption both in vitro and in vivo. J. Bone Miner. Res. 11, 1619–1627 (1996).
Chen, M. et al. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J. Exp. Med. 203, 837–842 (2006).
Griffiths, R. J. et al. Collagen-induced arthritis is reduced in 5-lipoxygenase-activating protein-deficient mice. J. Exp. Med. 185, 1123–1129 (1997).
Griffiths, R. J. et al. Leukotriene B4 plays a critical role in the progression of collagen-induced arthritis. Proc. Natl Acad. Sci. USA 92, 517–521 (1995).
Hashimoto, A. et al. Antiinflammatory mediator lipoxin A4 and its receptor in synovitis of patients with rheumatoid arthritis. J. Rheumatol. 34, 2144–2153 (2007).
Gheorghe, K. R. et al. Expression of 5-lipoxygenase and 15-lipoxygenase in rheumatoid arthritis synovium and effects of intraarticular glucocorticoids. Arthritis Res. Ther. 11, R83 (2009).
Leroux, J. L., Damon, M., Chavis, C., Crastes de Paulet, A. & Blotman, F. Effects of a single dose of methotrexate on 5- and 12-lipoxygenase products in patients with rheumatoid arthritis. J. Rheumatol. 19, 863–866 (1992).
Sperling, R. I. et al. Acute and chronic suppression of leukotriene B4 synthesis ex vivo in neutrophils from patients with rheumatoid arthritis beginning treatment with methotrexate. Arthritis Rheum. 35, 376–384 (1992).
Weinblatt, M. E. et al. Zileuton, a 5-lipoxygenase inhibitor in rheumatoid arthritis. J. Rheumatol. 19, 1537–1541 (1992).
Diaz-Gonzalez, F. et al. Clinical trial of a leucotriene B4 receptor antagonist, BIIL 284, in patients with rheumatoid arthritis. Ann. Rheum. Dis. 66, 628–632 (2007).
Liagre, B., Vergne, P., Rigaud, M. & Beneytout, J. L. Arachidonate 15-lipoxygenase of reticulocyte-type in human rheumatoid arthritis type B synoviocytes and modulation of its activity by proinflammatory cytokines. J. Rheumatol. 26, 1044–1051 (1999).
Deleuran, B. et al. Interleukin-8 secretion and 15-lipoxygenase activity in rheumatoid arthritis: in vitro anti-inflammatory effects by interleukin-4 and interleukin-10, but not by interleukin-1 receptor antagonist protein. Br. J. Rheum. 33, 520–525 (1994).
Profita, M. et al. Leukotriene B4 production in human mononuclear phagocytes is modulated by interleukin-4-induced 15-lipoxygenase. J. Pharmacol. Exp. Ther. 300, 868–875 (2002).
Ternowitz, T., Fogh, K. & Kragballe, K. 15-Hydroxyeicosatetraenoic acid (15-HETE) specifically inhibits LTB4-induced chemotaxis of human neutrophils. Skin Pharmacol. 1, 93–99 (1988).
Kronke, G., Uderhardt, S., Katzenbeisser, J. & Schett, G. The 12/15-lipoxygenase pathway promotes osteoclast development and differentiation. Autoimmunity 42, 383–385 (2009).
Sodin-Semrl, S., Taddeo, B., Tseng, D., Varga, J. & Fiore, S. Lipoxin A4 inhibits IL-1 β-induced IL-6, IL-8, and matrix metalloproteinase-3 production in human synovial fibroblasts and enhances synthesis of tissue inhibitors of metalloproteinases. J. Immunol. 164, 2660–2666 (2000).
Brash, A. R., Boeglin, W. E. & Chang, M. S. Discovery of a second 15S-lipoxygenase in humans. Proc. Natl Acad. Sci. USA 94, 6148–6152 (1997).
Giera, M. et al. Lipid and lipid mediator profiling of human synovial fluid in rheumatoid arthritis patients by means of LC-MS/MS. Biochim. Biophys. Acta 1821, 1415–1424 (2012).
Kronke, G. et al. 12/15-lipoxygenase counteracts inflammation and tissue damage in arthritis. J. Immunol. 183, 3383–3389 (2009).
Zhang, L. et al. BML-111, a lipoxin receptor agonist, modulates the immune response and reduces the severity of collagen-induced arthritis. Inflam. Res. 57, 157–162 (2008).
Lima-Garcia, J. F. et al. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br. J. Pharm. 164, 278–293 (2011).
Feltenmark, S. et al. Eoxins are proinflammatory arachidonic acid metabolites produced via the 15-lipoxygenase-1 pathway in human eosinophils and mast cells. Proc. Natl Acad. Sci. USA 105, 680–685 (2008).
Loeser, R. F., Goldring, S. R., Scanzello, C. R. & Goldring, M. B. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 64, 1697–1707 (2012).
Laufer, S. Role of eicosanoids in structural degradation in osteoarthritis. Curr. Opin. Rheumatol. 15, 623–627 (2003).
He, W., Pelletier, J. P., Martel-Pelletier, J., Laufer, S. & Di Battista, J. A. Synthesis of interleukin 1beta, tumor necrosis factor-alpha, and interstitial collagenase (MMP-1) is eicosanoid dependent in human osteoarthritis synovial membrane explants: interactions with antiinflammatory cytokines. J. Rheumatol. 29, 546–553 (2002).
Sahap Atik, O. Leukotriene B4 and prostaglandin E2-like activity in synovial fluid in osteoarthritis. Prostaglandins Leukot. Essent. Fatty Acids 39, 253–254 (1990).
Wittenberg, R. H., Willburger, R. E., Kleemeyer, K. S. & Peskar, B. A. In vitro release of prostaglandins and leukotrienes from synovial tissue, cartilage, and bone in degenerative joint diseases. Arthritis Rheum. 36, 1444–1450 (1993).
Attur, M., Dave, M., Abramson, S. B. & Amin, A. Activation of diverse eicosanoid pathways in osteoarthritic cartilage: a lipidomic and genomic analysis. Bull. NYU Hosp. Joint Dis. 70, 99–108 (2012).
Masuko-Hongo, K. et al. Up-regulation of microsomal prostaglandin E synthase 1 in osteoarthritic human cartilage: critical roles of the ERK-1/2 and p38 signaling pathways. Arthritis Rheum. 50, 2829–2838 (2004).
Kojima, F., Kato, S. & Kawai, S. Prostaglandin E synthase in the pathophysiology of arthritis. Fundam. Clin. Pharmacol. 19, 255–261 (2005).
Gosset, M. et al. Crucial role of visfatin/pre-B cell colony-enhancing factor in matrix degradation and prostaglandin E2 synthesis in chondrocytes: possible influence on osteoarthritis. Arthritis Rheum. 58, 1399–1409 (2008).
Zayed, N. et al. Increased expression of lipocalin-type prostaglandin D2 synthase in osteoarthritic cartilage. Arthritis Res. Ther. 10, R146 (2008).
Zayed, N. et al. Inhibition of interleukin-1beta-induced matrix metalloproteinases 1 and 13 production in human osteoarthritic chondrocytes by prostaglandin D2. Arthritis Rheum. 58, 3530–3540 (2008).
Martel-Pelletier, J. et al. Regulation of the expression of 5-lipoxygenase-activating protein/5-lipoxygenase and the synthesis of leukotriene B4 in osteoarthritic chondrocytes: role of transforming growth factor beta and eicosanoids. Arthritis Rheum. 50, 3925–3933 (2004).
Paredes, Y. et al. Study of the role of leukotriene B()4 in abnormal function of human subchondral osteoarthritis osteoblasts: effects of cyclooxygenase and/or 5-lipoxygenase inhibition. Arthritis Rheum. 46, 1804–1812 (2002).
Alvarez-Soria, M. A. et al. Long-term NSAID treatment directly decreases COX-2 and mPGES-1 production in the articular cartilage of patients with osteoarthritis. Osteoarthritis Cartil. 16, 1484–1493 (2008).
Faour, W. H. et al. Prostaglandin E2 regulates the level and stability of cyclooxygenase-2 mRNA through activation of p38 mitogen-activated protein kinase in interleukin-1 beta-treated human synovial fibroblasts. J. Biol. Chem. 276, 31720–31731 (2001).
Marcouiller, P. et al. Leukotriene and prostaglandin synthesis pathways in osteoarthritic synovial membranes: regulating factors for interleukin 1beta synthesis. J. Rheumatol. 32, 704–712 (2005).
Raynauld, J. P. et al. Protective effects of licofelone, a 5-lipoxygenase and cyclo-oxygenase inhibitor, versus naproxen on cartilage loss in knee osteoarthritis: a first multicentre clinical trial using quantitative MRI. Ann. Rheum. Dis. 68, 938–947 (2009).
Chini, M. G. et al. Design and synthesis of a second series of triazole-based compounds as potent dual mPGES-1 and 5-lipoxygenase inhibitors. Eur. J. Med. Chem. 54, 311–323 (2012).
Chabane, N. et al. Human articular chondrocytes express 15-lipoxygenase-1 and -2: potential role in osteoarthritis. Arthritis Res. Ther. 11, R44 (2009).
Prisk, V. & Huard, J. Muscle injuries and repair: the role of prostaglandins and inflammation. Histol. Histopathol. 18, 1243–1256 (2003).
Studynkova, J. T. et al. The expression of cyclooxygenase-1, cyclooxygenase-2 and 5-lipoxygenase in inflammatory muscle tissue of patients with polymyositis and dermatomyositis. Clin. Exp. Rheumatol. 22, 395–402 (2004).
Loell, I. et al. Activated LTB4 pathway in muscle tissue of patients with polymyositis or dermatomyositis. Ann. Rheum. Dis. 72, 293–299 (2013).
Choi, J., Kim, S. T. & Craft, J. The pathogenesis of systemic lupus erythematosus-an update. Curr. Opin. Immunol. 24, 651–657 (2012).
Patrono, C. et al. Functional significance of renal prostacyclin and thromboxane A2 production in patients with systemic lupus erythematosus. J. Clin. Invest. 76, 1011–1018 (1985).
Yoshida, T., Ichikawa, Y., Tojo, T. & Homma, M. Abnormal prostanoid metabolism in lupus nephritis and the effects of a thromboxane A2 synthetase inhibitor, DP-1904. Lupus 5, 129–138 (1996).
Avalos, I. et al. Aspirin therapy and thromboxane biosynthesis in systemic lupus erythematosus. Lupus 16, 981–986 (2007).
Steinhauer, H. B., Batsford, S., Schollmeyer, P. & Kluthe, R. Studies on thromboxane B2 and prostaglandin E2 production in the course of murine autoimmune disease: inhibition by oral histidine and zinc supplementation. Clin. Nephrol. 24, 63–68 (1985).
Spurney, R. F., Bernstein, R. J., Ruiz, P., Pisetsky, D. S. & Coffman, T. M. Physiologic role for enhanced renal thromboxane production in murine lupus nephritis. Prostaglandins 42, 15–28 (1991).
Poleshuck, L. C., Gartner, S. M., Belisle, E. H. & Strausser, H. R. Altered immune complex-induced prostaglandin production in human and murine lupus. Prostaglandins Med. 6, 317–327 (1981).
Chae, B. S. et al. Prostaglandin E2-mediated dysregulation of proinflammatory cytokine production in pristane-induced lupus mice. Arch. Pharm. Res. 31, 503–510 (2008).
Woolf, A. D. & Dieppe, P. A. Mediators of crystal-induced inflammation in the joint. Br. Med. Bull. 43, 429–444 (1987).
Bouchard, L., de Medicis, R., Lussier, A., Naccache, P. H. & Poubelle, P. E. Inflammatory microcrystals alter the functional phenotype of human osteoblast-like cells in vitro: synergism with IL-1 to overexpress cyclooxygenase-2. J. Immunol. 168, 5310–5317 (2002).
Bomalaski, J. S., Baker, D. G., Brophy, L. M. & Clark, M. A. Monosodium urate crystals stimulate phospholipase A2 enzyme activities and the synthesis of a phospholipase A2-activating protein. J. Immunol. 145, 3391–3397 (1990).
Pouliot, M., James, M. J., McColl, S. R., Naccache, P. H. & Cleland, L. G. Monosodium urate microcrystals induce cyclooxygenase-2 in human monocytes. Blood 91, 1769–1776 (1998).
Fam, A. G. Treating acute gouty arthritis with selective COX 2 inhibitors. Br. Med. J. 325, 980–981 (2002).
Schumacher, H. R. Jr et al. Randomised double blind trial of etoricoxib and indometacin in treatment of acute gouty arthritis. Br. Med. J. 324, 1488–1492 (2002).
Akahoshi, T. et al. Rapid induction of peroxisome proliferator-activated receptor gamma expression in human monocytes by monosodium urate monohydrate crystals. Arthritis Rheum. 48, 231–239 (2003).
Murakami, Y. et al. Inhibition of monosodium urate monohydrate crystal-induced acute inflammation by retrovirally transfected prostaglandin D synthase. Arthritis Rheum. 48, 2931–2941 (2003).
Jung, S. M. et al. Reduction of urate crystal-induced inflammation by root extracts from traditional oriental medicinal plants: elevation of prostaglandin D2 levels. Arthritis Res. Ther. 9, R64 (2007).
Rae, S. A., Davidson, E. M. & Smith, M. J. Leukotriene B4, an inflammatory mediator in gout. Lancet 2, 1122–1124 (1982).
Leslie, C. A. et al. Dietary fish oil modulates macrophage fatty acids and decreases arthritis susceptibility in mice. J. Exp. Med. 162, 1336–1349 (1985).
Lee, T. H. et al. Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. N. Engl. J. Med. 312, 1217–1224 (1985).
Bagga, D., Wang, L., Farias-Eisner, R., Glaspy, J. A. & Reddy, S. T. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc. Natl Acad. Sci. USA 100, 1751–1756 (2003).
Serhan, C. N. & Chiang, N. Resolution phase lipid mediators of inflammation: agonists of resolution. Curr. Opin. Pharmacol. 13, 632–640 (2013).
Calder, P. C. Omega-3 fatty acids and inflammatory processes. Nutrients 2, 355–374 (2010).
Ierna, M., Kerr, A., Scales, H., Berge, K. & Griinari, M. Supplementation of diet with krill oil protects against experimental rheumatoid arthritis. BMC Musculoskelet. Disord. 11, 136 (2010).
Alexander, N. J., Smythe, N. L. & Jokinen, M. P. The type of dietary fat affects the severity of autoimmune disease in NZB/NZW mice. Am. J. Pathol. 127, 106–121 (1987).
Halade, G. V., Williams, P. J., Veigas, J. M., Barnes, J. L. & Fernandes, G. Concentrated fish oil (Lovaza®) extends lifespan and attenuates kidney disease in lupus-prone short-lived (NZBxNZW)F1 mice. Exp. Biol. Med. 238, 610–622 (2013).
James, M., Proudman, S. & Cleland, L. Fish oil and rheumatoid arthritis: past, present and future. Proc. Nutr. Soc. 69, 316–323 (2010).
Miles, E. A. & Calder, P. C. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br. J. Nutr. 107 (Suppl. 2), S171–S184 (2012).
Duffy, E. M. et al. The clinical effect of dietary supplementation with omega-3 fish oils and/or copper in systemic lupus erythematosus. J. Rheumatol. 31, 1551–1556 (2004).
Acknowledgements
P.-J.J and M.K. would like to acknowledge support by The Swedish Research Council, The Swedish Rheumatism Association, King Gustaf V 80 years Foundation, The Swedish Society of Medicine, Karolinska Institutet Foundation, The Swedish County Council and Marianne and Marcus Wallenberg foundation.
Author information
Authors and Affiliations
Contributions
M.K. researched data for the article. Both authors contributed equally to discussion of content, writing, and reviewing/editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
P.-J.J. is a member of the board of directors for NovaSAID AB. M.K. declares no competing interests.
Supplementary information
Supplementary Table 1
A list of the described eicosanoids and their receptors (DOC 54 kb)
PowerPoint slides
Rights and permissions
About this article
Cite this article
Korotkova, M., Jakobsson, PJ. Persisting eicosanoid pathways in rheumatic diseases. Nat Rev Rheumatol 10, 229–241 (2014). https://doi.org/10.1038/nrrheum.2014.1
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2014.1
This article is cited by
-
Lipidome profile predictive of disease evolution and activity in rheumatoid arthritis
Experimental & Molecular Medicine (2022)
-
Differences in oxylipin profile in psoriasis versus psoriatic arthritis
Arthritis Research & Therapy (2021)
-
Higher PGD2 production by synovial mast cells from rheumatoid arthritis patients compared with osteoarthritis patients via miR-199a-3p/prostaglandin synthetase 2 axis
Scientific Reports (2021)
-
Prophylactic effects of probiotic Bifidobacterium spp. in the resolution of inflammation in arthritic rats
Applied Microbiology and Biotechnology (2019)
-
Pro- and anti-inflammatory eicosanoids in psoriatic arthritis
Metabolomics (2019)