Key Points
-
Rheumatoid arthritis (RA) is caused by an intricate interplay between T cells, macrophages and B cells; these cells or their products are, therefore, important targets of therapy
-
T-cell directed approaches target co-stimulation as well as molecules involved in T-cell activation and regulation (such as CD4 antigen)
-
Novel therapeutic approaches might include harnessing the action of regulatory T cells
-
Compounds targeting GM-CSF and IL-17 are already in an advanced stage of clinical development in RA
-
Cytokines such as IL-20 and IL-21 contribute to the pathogenesis of RA and have promise as potential targets for treatment
-
B-cell directed therapies comprise anti-CD20 therapies, CD19-directed and CD52-directed depletion strategies; alternative approaches include CD22-mediated ligation of inhibitory B-cell receptor and modification of adhesion molecule expression by B cells
Abstract
Despite major progress in the treatment of rheumatoid arthritis (RA), strong unmet medical need remains, as only a minor proportion of patients reach sustained clinical remission. New approaches are therefore necessary, and include manipulation of regulatory T cells, which might be able to restore the disturbed immune system and could even lead to a cure if this restored regulation were to prove sustainable. Logistical and conceptual problems, however, beset this attractive therapeutic approach, including difficulties with ex vivo expansion of cells, specificity of targeting and the optimal time point of administration. Therefore, alternative avenues are being investigated, such as targeting B-cell effector functions and newly identified proinflammatory cytokines. On the basis of success with B-cell depleting therapy using anti-CD20 agents, further treatment modalities are now exploring direct or indirect interference in B-cell-mediated immunity with the use of agents directed against other B-cell surface molecules. Novel approaches target intracellular B-cell signalling and regulatory B cells. New cytokine-directed therapies target important proinflammatory mediators such as GM-CSF, new members of the IL-1 family, IL-6 and its receptor, IL-17, IL-20, IL-21, IL-23 as well as synovium-specific targets. This article reviews these emerging cell and cytokine targets with special focus on biologic agents, some of which might reach the clinic soon whereas others will require considerable time in development. Nevertheless, these exciting new approaches will considerably enhance our repertoire in the battle against this potentially devastating disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Burmester, G. R., Pratt, A. G., Scherer, H. U. & van Laar, J. M. in EULAR Textbook on Rheumatic Diseases 1st edn, Ch. 9. Rheumatoid arthritis: pathogenesis and clinical features (ed. Bijlsma, J. W.), 206–231 (BMJ Group, London, 2012).
Cope, A. P, Schulze-Koops, H. & Aringer, M. The central role of T cells in rheumatoid arthritis. Clin. Exp. Rheumatol. 25 (Suppl. 46), S4–S11 (2007).
Komatsu, N. & Takayanagi, H. Autoimmune arthritis: the interface between the immune system and joints. Adv. Immunol. 115, 45–71 (2012).
Ornstein, M. H., Kerr, L. D. & Spiera H. A reexamination of the relationship between active rheumatoid arthritis and the acquired immunodeficiency syndrome. Arthritis Rheum. 38, 1701–1706 (1995).
Fiocco, U. et al. Co-stimulatory modulation in rheumatoid arthritis: the role of (CTLA4-Ig) abatacept. Autoimmun. Rev. 8, 76–82 (2008).
Martinez-Gamboa, L., Brezinschek, H. P., Burmester, G. R. & Dörner, T. Immunopathologic role of B lymphocytes in rheumatoid arthritis: rationale of B cell-directed therapy. Autoimmun. Rev. 5, 437–442 (2006).
Burmester, G. R. et al. The tissue architecture of synovial membranes in inflammatory and non-inflammatory joint diseases. I. The localization of the major synovial cell populations as detected by monoclonal reagents directed towards Ia and monocyte-macrophage antigens. Rheumatol. Int. 3, 173–181 (1983).
McInnes, I. B. & Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365, 2205–2219 (2011).
Redlich, K. & Smolen, J. S. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat. Rev. Drug Disc. 11, 234–250 (2012).
Smolian, H. et al. Secretion of gelatinases and activation of gelatinase A (MMP-2) by human rheumatoid synovial fibroblasts. Biol. Chem. 382, 1491–1499 (2001).
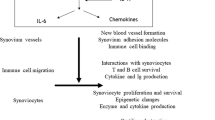
Neumann, E., Lefèvre, S., Zimmermann, B., Gay, S. & Müller-Ladner, U. Rheumatoid arthritis progression mediated by activated synovial fibroblasts. Trends Mol. Med. 16, 458–468 (2010).
McInnes, I. B. & Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 7, 429–442 (2007).
Smolen, J. S., Aletaha, D. & Redlich, K. The pathogenesis of rheumatoid arthritis: new insights from old clinical data? Nat. Rev. Rheumatol. 8, 235–243 (2012).
Singh, J. A. et al. A network meta-analysis of randomized controlled trials of biologics for rheumatoid arthritis: a Cochrane overview. CMAJ 181, 787–796 (2009).
Salliot, C. et al. Indirect comparisons of the efficacy of biological antirheumatic agents in rheumatoid arthritis in patients with an inadequate response to conventional disease-modifying antirheumatic drugs or to an anti-tumour necrosis factor agent: a meta-analysis. Ann. Rheum. Dis. 70, 266–271 (2011).
Simard, J. F. et al. Ten years with biologics: to whom do data on effectiveness and safety apply? Rheumatology (Oxford) 50, 204–213 (2011).
O'Shea, J. J., Holland, S. M. & Staudt, L. M. JAKs and STATs in immunity, immunodeficiency, and cancer. N. Engl. J. Med. 368, 161–170 (2013).
Feist, E. & Burmester, G. R. Small molecules targeting JAKs--a new approach in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 52, 1352–1357 (2013).
Mauri, C. & Carter, N. Is there a feudal hierarchy amongst regulatory immune cells? More than just TREGs. Arthritis Res. Ther. 11, 237 (2009).
Buckner, J. H. Mechanisms of impaired regulation by CD4+CD25+FOXP3+ regulatory T cells in human autoimmune diseases. Nat. Rev. Immunol. 10, 849–859 (2010).
Ochs, H. D., Gambineri, E. & Torgerson, T. R. IPEX, FOXP3 and regulatory T-cells: a model for autoimmunity. Immunol. Res. 38, 112–121 (2007).
Morgan, M. E. et al. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 48, 1452–1460 (2003).
Frey, O. et al. The role of regulatory T cells in antigen-induced arthritis: aggravation of arthritis after depletion and amelioration after transfer of CD4+CD25+ T cells. Arthritis Res. Ther. 7, R291–R301 (2005).
Kong, N. et al. Induced T regulatory cells suppress osteoclastogenesis and bone erosion in collagen-induced arthritis better than natural T regulatory cells. Ann. Rheum. Dis. 71, 1567–1572 (2012).
Alexander, T. et al. FOXP3+ Helios+ regulatory T cells are expanded in active systemic lupus erythematosus. Ann. Rheum. Dis. 72, 1549–1558 (2013).
Zhou, X., Bailey-Bucktrout, S., Jeker, L. T. & Bluestone, J. A. Plasticity of CD4+ FOXP3+ T cells. Curr. Opin. Immunol. 21, 281–285 (2009).
Esensten, J. H., Wofsy, D. & Bluestone, J. A. Regulatory T cells as therapeutic targets in rheumatoid arthritis. Nat. Rev. Rheumatol. 5, 560–565 (2009).
Cao, D. et al. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur. J. Immunol. 33, 215–223 (2003).
Möttönen, M. et al. CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin. Exp. Immunol. 140, 360–367 (2005).
Lawson, C. A. et al. Early rheumatoid arthritis is associated with a deficit in the CD4+CD25high regulatory T cell population in peripheral blood. Rheumatology (Oxford) 45, 1210–1217 (2006).
Han, G. M. O'Neil-Andersen, N. J., Zurier, R. B. & Lawrence, D. A. CD4+CD25high T cell numbers are enriched in the peripheral blood of patients with rheumatoid arthritis. Cell. Immunol. 253, 92–101 (2008).
Ehrenstein, M. R. et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFα therapy. J. Exp. Med. 200, 277–285 (2004).
Klareskog, L., Rönnelid, J., Lundberg, K., Padyukov, L. & Alfredsson, L. Immunity to citrullinated proteins in rheumatoid arthritis. Annu. Rev. Immunol. 26, 651–675 (2008).
Skriner, K. et al. Anti-A2/RA33 autoantibodies are directed to the RNA binding region of the A2 protein of the heterogeneous nuclear ribonucleoprotein complex. Differential epitope recognition in rheumatoid arthritis, systemic lupus erythematosus, and mixed connective tissue disease. J. Clin. Invest. 100, 127–135 (1997).
Wright, G. P., Stauss, H. J. & Ehrenstein, M. R. Therapeutic potential of TREGs to treat rheumatoid arthritis. Semin. Immunol. 23, 195–201 (2011).
Nielen, M. M. et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 50, 380–386 (2004).
Koreth, J. et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N. Engl. J. Med. 365, 2055–2066 (2011).
Saadoun, D. et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N. Engl. J. Med. 365, 2067–2077 (2011).
Czeloth, N. et al. Selective activation of naturally occurring regulatory T cells (TREGs) by the monoclonal antibody BT-061 as a novel therapeutic opportunity: pre-clinical and early clinical results [abstract OP0138]. Ann. Rheum. Dis. 69 (Suppl. 3), 99 (2010).
Uherek, C. et al. The novel regulatory T cell (TREG) agonistic monoclonal antibody (mAb) tregalizumab (BT-061): further characterization of mechanism of action, epitope binding, and clinical effects in patients with rheumatoid arthritis. www.biotest.de[online] (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2011).
Cousens, L. P. et al. Tregitope update: Mechanism of action parallels IVIg. Autoimmun. Rev. 12, 436–443 (2013).
Dörner, T., Kinnman, N. & Tak, P. P. Targeting B cells in immune-mediated inflammatory disease: a comprehensive review of mechanisms of action and identification of biomarkers. Pharmacol. Ther. 125, 464–475 (2010).
Dörner, T., Radbruch, A. & Burmester, G. R. B-cell-directed therapies for autoimmune disease. Nat. Rev. Rheumatol. 5, 433–441 (2009).
Klareskog, L., Widhe, M., Hermansson, M. & Rönnelid, J. Antibodies to citrullinated proteins in arthritis: pathology and promise. Curr. Opin. Rheumatol. 20, 300–305 (2008).
Amara, K. et al. Monoclonal IgG antibodies generated from joint-derived B cells of RA patients have a strong bias toward citrullinated autoantigen recognition. J. Exp. Med. 210, 445–455 (2013).
Edwards, J. C. et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N. Engl. J. Med. 350, 2572–2581 (2004).
Emery, P. et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum. 54, 1390–1400 (2006).
Cohen, S. B. et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 54, 2793–2806 (2006).
Jones, R. B. et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N. Engl. J. Med. 363, 211–220 (2010).
Glennie, M. J., French, R. R., Cragg, M. S. & Taylor, R. P. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol. Immunol. 44, 3823–3837 (2007).
Alduaij, W. et al. Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosome-mediated cell death in B-cell malignancies. Blood 117, 4519–4529 (2011).
Cragg, M. S. & Glennie, M. J. Antibody specificity controls in vivo effector mechanisms of anti-CD20 reagents. Blood 103, 2738–2743 (2004).
Mei, H. E., Schmidt, S. & Dörner T. Rationale of anti-CD19 immunotherapy: an option to target autoreactive plasma cells in autoimmunity. Arthritis Res. Ther. 14 (Suppl. 5), S1 (2012).
Roschke, V. et al. BLyS and APRIL form biologically active heterotrimers that are expressed in patients with systemic immune-based rheumatic diseases. J. Immunol. 169, 4314–4321 (2002).
Stashenko, P., Nadler, L. M., Hardy, R. & Schlossman, S. F. Characterization of a human B lymphocyte-specific antigen. J. Immunol. 125, 1678–1685 (1980).
Stashenko, P., Nadler, L. M., Hardy, R. & Schlossman, S. F. Expression of cell surface markers after human B lymphocyte activation. Proc. Natl Acad. Sci. USA 78, 3848–3852 (1981).
Horton, H. M. et al. Potent in vitro and in vivo activity of an Fc-engineered anti-CD19 monoclonal antibody against lymphoma and leukemia. Cancer Res. 68, 8049–8057 (2008).
Bargou, R. et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 321, 974–977 (2008).
Sato, S., Ono, N., Steeber, D. A., Pisetsky, D. S. & Tedder, T. F. CD19 regulates B lymphocyte signaling thresholds critical for the development of B-1 lineage cells and autoimmunity. J. Immunol. 157, 4371–4378 (1996).
Daridon, C. et al. Epratuzumab targeting of CD22 affects adhesion molecule expression and migration of B-cells in systemic lupus erythematosus. Arthritis Res. Ther. 12, R204 (2010).
Tedder, T. F., Poe, J. C. & Haas, K. M. CD22: a multifunctional receptor that regulates B lymphocyte survival and signal transduction. Adv. Immunol. 88, 1–50 (2005).
Jacobi, A. M. et al. Differential effects of epratuzumab on peripheral blood B cells of patients with systemic lupus erythematosus versus normal controls. Ann. Rheum. Dis. 67, 450–457 (2008).
Sieger, N. et al. CD22 ligation inhibits downstream B-cell receptor signaling and Ca2+ flux upon activation. Arthritis Rheum. 65, 770–779 (2013).
Dörner, T. & Burmester, G. R. The role of B cells in rheumatoid arthritis: mechanisms and therapeutic targets. Curr. Opin. Rheumatol. 15, 246–252 (2003).
Buch, M. H. et al. Updated consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann. Rheum. Dis. 70, 909–920 (2011).
Stohl, W. et al. Safety and efficacy of ocrelizumab in combination with methotrexate in MTX-naive subjects with rheumatoid arthritis: the phase III FILM trial. Ann. Rheum. Dis. 71, 1289–1296 (2012).
Tak, P. P. et al. Safety and efficacy of ocrelizumab in patients with rheumatoid arthritis and an inadequate response to at least one tumor necrosis factor inhibitor: results of a forty-eight-week randomized, double-blind, placebo-controlled, parallel-group phase III trial. Arthritis Rheum. 64, 360–370 (2012).
Østergaard, M. et al. Ofatumumab, a human anti-CD20 monoclonal antibody, for treatment of rheumatoid arthritis with an inadequate response to one or more disease-modifying antirheumatic drugs: results of a randomized, double-blind, placebo-controlled, phase I/II study. Arthritis Rheum. 62, 2227–2238 (2010).
Taylor, P. C. et al. Ofatumumab, a fully human anti-CD20 monoclonal antibody, in biological-naive, rheumatoid arthritis patients with an inadequate response to methotrexate: a randomised, double-blind, placebo-controlled clinical trial. Ann. Rheum. Dis. 70, 2119–2125 (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Isaacs, J. D. et al. CAMPATH-1H in rheumatoid arthritis--an intravenous dose-ranging study. Br. J. Rheumatol. 35, 231–240 (1996).
Lorenzi, A. R. et al. Morbidity and mortality in rheumatoid arthritis patients with prolonged therapy-induced lymphopenia: twelve-year outcomes. Arthritis Rheum. 58, 370–375 (2008).
Brown, J. & Coles, A. J. Alemtuzumab: evidence for its potential in relapsing-remitting multiple sclerosis. Drug Des. Devel. Ther. 7, 131–118 (2013).
Groom, J. et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren's syndrome. J. Clin. Invest. 109, 59–68 (2002).
Ohata, J. et al. Fibroblast-like synoviocytes of mesenchymal origin express functional B cell-activating factor of the TNF family in response to proinflammatory cytokines. J. Immunol. 174, 864–870 (2005).
Gross, J. A. et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature 404, 995–999 (2000).
Bajpai, M., Chopra, P., Dastidar, S. G. & Ray, A. Spleen tyrosine kinase: a novel target for therapeutic intervention of rheumatoid arthritis. Expert Opin. Investig. Drugs. 17, 641–659 (2008).
Lindstrom, T. M. & Robinson, W. H. A multitude of kinases—which are the best targets in treating rheumatoid arthritis? Rheum. Dis. Clin. North Am. 36, 367–383 (2010).
Mauri, C. & Blair, P. A. Regulatory B cells in autoimmunity: developments and controversies. Nat. Rev. Rheumatol. 6, 636–643 (2010).
Iwata, Y. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 117, 530–541 (2011).
Flores-Borja, F. et al. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci. Transl. Med. 5, 173ra23 (2013).
Hamilton, J. A. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 8, 533–544 (2008).
Berenbaum, F., Rajzbaum, G., Amor, B. & Toubert, A. Evidence for GM-CSF receptor expression in synovial tissue. An analysis by semi-quantitative polymerase chain reaction on rheumatoid arthritis and osteoarthritis synovial biopsies. Eur. Cytokine Netw. 5, 43–46 (1994).
Wang, T., Lazar, C. A., Fishbein, M. C. & Lynch, J. P. 3rd. Pulmonary alveolar proteinosis. Semin. Respir. Crit. Care Med. 33, 498–508 (2012).
Khan, A. & Agarwal, R. Pulmonary alveolar proteinosis. Respir. Care 56, 1016–1028 (2011).
Hansen, G. et al. The structure of the GM-CSF receptor complex reveals a distinct mode of cytokine receptor activation. Cell 134, 496–507 (2008).
Burmester, G. R. et al. Mavrilimumab, a human monoclonal antibody targeting GM-CSF receptor-α, in subjects with rheumatoid arthritis: a randomised, double-blind, placebo-controlled, phase I, first-in-human study. Ann. Rheum. Dis. 70, 1542–1549 (2011).
Burmester, G. R. et al. Efficacy and safety of mavrilimumab in subjects with rheumatoid arthritis. Ann. Rheum. Dis. 72, 1445–1452 (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
Gaffen, S. L. An overview of IL-17 function and signaling. Cytokine 43, 402–407 (2008).
Miossec, P., Korn, T. & Kuchroo, V. K. Interleukin-17 and type 17 helper T cells. N. Engl. J. Med. 361, 888–898 (2009).
Yang, J. et al. TH17 and natural TREG cell population dynamics in systemic lupus erythematosus. Arthritis Rheum. 60, 1472–1483 (2009).
Kwan, B. C. et al. The gene expression of type 17 T-helper cell-related cytokines in the urinary sediment of patients with systemic lupus erythematosus. Rheumatology (Oxford) 48, 1491–1497 (2009).
Wang, Y. et al. Laser microdissection-based analysis of cytokine balance in the kidneys of patients with lupus nephritis. Clin. Exp. Immunol. 159, 1–10 (2010).
Nistala, K. et al. TH17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc. Natl Acad. Sci. USA 107, 14751–14756 (2010).
Redlich, K. & Smolen, J. S. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 11, 234–250 (2012).
Hueber, W. et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci. Transl. Med. 2, 52ra72 (2010).
Genovese, M. C. et al. Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled study. Ann. Rheum. Dis. 72, 863–869 (2013).
Rich, P. et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br. J. Dermatol. 168, 402–411 (2013).
Hueber, W. et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61, 1693–1700 (2012).
Leonardi, C. et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N. Engl. J. Med. 366, 1190–1199 (2012).
Genovese, M. C. et al. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum. 62, 929–939 (2010).
Genovese, M. et al. A phase 2 study of multiple subcutaneous doses of LY2439821, an anti-IL-17 monoclonal antibody, in patients with rheumatoid arthritis in two populations: naïve to biologic therapy or inadequate responders to tumor necrosis factor α inhibitors [abstract]. Arthritis Rheum. 63 (Suppl. 10) 2591 (2011).
Hsu, Y. H. et al. Function of interleukin-20 as a proinflammatory molecule in rheumatoid and experimental arthritis. Arthritis Rheum. 54, 2722–2733 (2006).
Kragstrup, T. W. et al. The expression of IL-20 and IL-24 and their shared receptors are increased in rheumatoid arthritis and spondyloarthropathy. Cytokine 41, 16–23 (2008).
Hsu, Y. H. Anti-IL-20 monoclonal antibody inhibits the differentiation of osteoclasts and protects against osteoporotic bone loss. J. Exp. Med. 208, 1849–1861 (2011).
Hsu, Y. H. et al. Anti-IL-20 monoclonal antibody inhibits the differentiation of osteoclasts and protects against osteoporotic bone loss. J. Exp. Med. 208, 1849–1861 (2011).
Al Kotbi, N., Jensen, L. & Graff, L. B. NNC0109–0012 (anti-IL-20 mAb), well tolerated in healthy subjects and patients with rheumatoid arthritis [abstract FRI0196]. Ann. Rheum. Dis. 71 (Suppl. 3), 379 (2012).
Leszczynski, P., Eshof, M. K., Stegmann, H. V. B., Hundahl Møller, N. P. & Graff, L. B. NNC0109–0012 (anti-IL-20 mAb), well tolerated in patients with rheumatoid arthritis [abstract FRI0197]. Ann. Rheum. Dis. 71 (Suppl. 3), 379 (2012).
Šenolt, L., Göthberg, M., Valencia, X. & Dokoupilová, E. Efficacy and safety of NNC0109–0012 (anti-IL-20 mAb) in patients with rheumatoid arthritis: results from a phase 2a trial [abstract LB0004]. Ann. Rheum. Dis. 71 (Suppl. 3), 152 (2012).
Kwok, S. K. et al. Interleukin-21 promotes osteoclastogenesis in humans with rheumatoid arthritis and in mice with collagen-induced arthritis. Arthritis Rheum. 64, 740–751 (2012).
Rasmussen, T. K. et al. Increased interleukin 21 (IL-21) and IL-23 are associated with increased disease activity and with radiographic status in patients with early rheumatoid arthritis. J. Rheumatol. 37, 2014–2020 (2010).
Raveney, B. J., Oki, S. & Yamamura, T. Nuclear receptor NR4A2 orchestrates TH17 cell-mediated autoimmune inflammation via IL-21 signalling. PLoS ONE 8, e56595 (2013).
Niu, X. et al. IL-21 regulates TH17 cells in rheumatoid arthritis. Hum. Immunol. 71, 334–341 (2010).
Gottenberg, J. E. et al. Serum IL-6 and IL-21 are associated with markers of B cell activation and structural progression in early rheumatoid arthritis: results from the ESPOIR cohort. Ann. Rheum. Dis. 71, 1243–1248 (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
Tak, P. P. et al. Chemokine receptor CCR1 antagonist CCX354-C treatment for rheumatoid arthritis: CARAT-2, a randomised, placebo controlled clinical trial. Ann. Rheum. Dis. 72, 337–344 (2013).
Filková, M., Jüngel, A., Gay, R. E. & Gay, S. MicroRNAs in rheumatoid arthritis: potential role in diagnosis and therapy. BioDrugs 26, 131–141 (2012).
Gregersen, J. W. & Jayne, D. R. B-cell depletion in the treatment of lupus nephritis Nat. Rev. Nephrol. 8, 505–514 (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Wallace, D.J. et al. Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study. Ann. Rheum. Dis. 10.1136/annrheumdis-2012-202760.
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Author information
Authors and Affiliations
Contributions
All author contributed substantially to each stage of the preparation of this manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
G. R. Burmester declares that he has received speakers' honoraria and has acted as a consultant for AbbVie, BMS, MedImmune, MSD, Pfizer, Roche/Chugai and UCB. E. Feist declares that he has received speakers' honoraria and has acted as a consultant for BMS, Pfizer, Novartis and Roche/Chugai. T. Dörner declares that he has received speakers' honoraria and has acted as a consultant for Elli Lilly, NovoNordisk, Roche/Chugai, Takeda and UCB, and has received research/grant support from Roche/Chugai, UCB, Takeda, Janssen/J&J and Sanofi.
Rights and permissions
About this article
Cite this article
Burmester, G., Feist, E. & Dörner, T. Emerging cell and cytokine targets in rheumatoid arthritis. Nat Rev Rheumatol 10, 77–88 (2014). https://doi.org/10.1038/nrrheum.2013.168
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2013.168
This article is cited by
-
Ceria-vesicle nanohybrid therapeutic for modulation of innate and adaptive immunity in a collagen-induced arthritis model
Nature Nanotechnology (2023)
-
Should We Use bDMARDs as an Induction Therapy in Early and Severe Rheumatoid Arthritis? Results at 5 years from the ERA UCLouvain Brussels Cohort
Rheumatology and Therapy (2023)
-
Inflammatory-associated apoptotic markers: are they the culprit to rheumatoid arthritis pain?
Molecular Biology Reports (2022)
-
Novel anti-arthritic mechanisms of trans-cinnamaldehyde against complete Freund’s adjuvant-induced arthritis in mice: involvement of NF-кB/TNF-α and IL-6/IL-23/ IL-17 pathways in the immuno-inflammatory responses
Inflammopharmacology (2022)
-
The Role of Cell Organelles in Rheumatoid Arthritis with Focus on Exosomes
Biological Procedures Online (2021)