Key Points
-
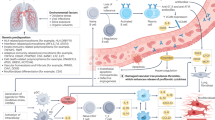
Extra-articular features, particularly pulmonary complications, are a major cause of mortality in autoimmune rheumatic disease
-
Interstitial lung disease (ILD) is an important pulmonary complication in a variety of connective tissues diseases, particularly systemic sclerosis
-
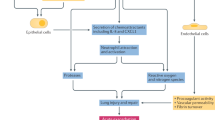
Despite advances in understanding of disease mechanisms, more work needs to be done to identify cardinal pathogenetic pathways in order to develop more efficacious therapies
-
The most difficult clinical decision in ILD associated with connective tissue disease is to determine when treatment should be initiated and when meticulous observation without intervention is appropriate
-
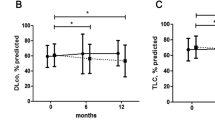
The initiation of treatment is warranted when disease progression is probable, based on pulmonary disease severity, evidence of ongoing progression and short duration of systemic disease
-
Current treatment is essentially based on immunosuppression: it is imperative that novel immunomodulatory and antifibrotic agents be evaluated in multicentre treatment trials
Abstract
Pulmonary complications are an important extra-articular feature of autoimmune rheumatic diseases and a major cause of mortality. The underlying pathogenesis probably involves multiple cellular compartments, including the epithelium, lung fibroblasts, and the innate and adaptive immune system. Heterogeneity in the extent and progression of lung fibrosis probably reflects differences in underlying pathogenic mechanisms. Growing understanding of the key pathogenic drivers of lung fibrosis might lead to the development of more effective targeted therapies to replicate the treatment advances in other aspects of these diseases. Interstitial lung disease (ILD) in connective tissue disease (CTD) is characterized using the classification of the idiopathic interstitial pneumonias. Systemic sclerosis is most frequently associated with ILD and, in most of these patients, ILD manifests as a histological pattern of nonspecific interstitial pneumonia. Conversely, in rheumatoid arthritis, the pattern of ILD is most often usual interstitial pneumonia. The key goals of clinical assessment of patients with both ILD and CTD are the detection of ILD and prognostic evaluation to determine which patients should be treated. Data from treatment trials in systemic sclerosis support the use of immunosuppressive therapy, with the treatment benefit largely relating to the prevention of progression of lung disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Fischer, A. & du Bois, R. Interstitial lung disease in connective tissue disorders. Lancet 380, 689–698 (2012).
Maurer, B. et al. Fra-2 transgenic mice as a novel model of pulmonary hypertension associated with systemic sclerosis. Ann. Rheum. Dis. 71, 1382–1387 (2012).
Hsu, E., Shi, H., Jordan, R. M., Lyons-Weiler, J., Pilewski, J. M. & Feghali-Bostwick, C. A. Lung tissues in patients with systemic sclerosis have gene expression patterns unique to pulmonary fibrosis and pulmonary hypertension. Arthritis Rheum. 63, 783–794 (2011).
Peljto, A. L. et al. The pulmonary fibrosis-associated MUC5B promoter polymorphism does not influence the development of interstitial pneumonia in systemic sclerosis. Chest 142, 1584–1588 (2012).
Stock, C. J. et al. Mucin 5B promoter polymorphism is associated with idiopathic pulmonary fibrosis but not with development of lung fibrosis in systemic sclerosis or sarcoidosis. Thorax 68, 436–441 (2013).
Hant, F. N. et al. Scleroderma Lung Study Research Group. Surfactant protein D and KL-6 as serum biomarkers of interstitial lung disease in patients with scleroderma. J. Rheumatol. 36, 773–780 (2009).
de Carvalho, E. F. et al. Arterial and interstitial remodelling processes in non-specific interstitial pneumonia: systemic sclerosis versus idiopathic. Histopathology 53, 195–204 (2008).
Hoyles, R. K. et al. Fibroblast-specific perturbation of transforming growth factor β signaling provides insight into potential pathogenic mechanisms of scleroderma-associated lung fibrosis: exaggerated response to alveolar epithelial injury in a novel mouse model. Arthritis Rheum. 58, 1175–1188 (2008).
Christmann, R. B., Wells, A. U., Capelozzi, V. L. & Silver, R. M. Gastroesophageal reflux incites interstitial lung disease in systemic sclerosis: clinical, radiologic, histopathologic, and treatment evidence. Semin. Arthritis Rheum. 40, 241–249 (2010).
Walker, N. et al. Resident tissue-specific mesenchymal progenitor cells contribute to fibrogenesis in human lung allografts. Am. J. Pathol. 178, 2461–2469 (2011).
Borie, R. et al. Detection of alveolar fibrocytes in idiopathic pulmonary fibrosis and systemic sclerosis. PLoS ONE 8, e53736 (2013).
Sonnylal, S. et al. Postnatal induction of transforming growth factor β signaling in fibroblasts of mice recapitulates clinical, histologic, and biochemical features of scleroderma. Arthritis Rheum. 56, 334–344 (2007).
Hoyles, R. K. et al. An essential role for resident fibroblasts in experimental lung fibrosis is defined by lineage-specific deletion of high-affinity type II transforming growth factor receptor. Am. J. Respir. Crit. Care Med. 183, 249–261 (2011).
Goh, N. S. et al. Increased epithelial permeability in pulmonary fibrosis in relation to disease progression. Eur. Respir. J. 38, 184–190 (2011).
Bartis, D., Mise, N., Mahida, R. Y., Eickelberg, O. & Thickett, D. R. Epithelial-mesenchymal transition in lung development and disease: does it exist and is it important? Thorax 69, 760–765 (2014).
Denton, C. P. et al. Activation of key profibrotic mechanisms in transgenic fibroblasts expressing kinase-deficient type II TGF-β receptor. J. Biol. Chem. 280, 16053–16065 (2005).
Denton, C. P. et al. Fibroblast-specific expression of a kinase-deficient type II transforming growth factor β receptor leads to paradoxical activation of TGFβ signaling pathways with fibrosis in transgenic mice. J. Biol. Chem. 278, 25109–25119 (2003).
Derrett-Smith, E. et al. Endothelial injury in a transforming growth factor β-dependent mouse model of scleroderma induces pulmonary arterial hypertension. Arthritis Rheum. 65, 2928–2239 (2013).
Nihtyanova, S. I. et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheumatol. 66, 1625–1635 (2014).
Nihtyanova, S. I. & Denton, C. P. Autoantibodies as predictive tools in systemic sclerosis. Nat. Rev. Rheumatol. 6, 112–116 (2010).
Giovannetti, A. et al. Analyses of T cell phenotype and function reveal an altered T cell homeostasis in systemic sclerosis. Correlations with disease severity and phenotypes. Clin. Immunol. 137, 122–133 (2010).
Pechkovsky, D. V. et al. Alternatively activated alveolar macrophages in pulmonary fibrosis-mediator production and intracellular signal transduction. Clin. Immunol. 137, 89–101 (2010).
Dieud, P. et al. NLRP1 influences the systemic sclerosis phenotype: a new clue for the contribution of innate immunity in systemic sclerosis-related fibrosing alveolitis pathogenesis. Ann. Rheum. Dis. 70, 668–674 (2011).
Beirne, P. et al. Multiplex immune serum biomarker profiling in sarcoidosis and systemic sclerosis. Eur. Respir. J. 34, 1376–1382 (2009).
Fonseca, C. et al. A polymorphism in the CTGF promoter region associated with systemic sclerosis. N. Engl. J. Med. 357, 1210–1220 (2007).
Bossini-Castillo, L. et al. A multicenter study confirms CD226 gene association with systemic sclerosis-related pulmonary fibrosis. Arthritis Res. Ther. 14, R85 (2012).
Gorlova, O. et al. Identification of novel genetic markers associated with clinical phenotypes of systemic sclerosis through a genome-wide association strategy. PLoS Genet. 7, e1002178 (2011).
Cénit, M. C. et al. Influence of the IL6 gene in susceptibility to systemic sclerosis. J. Rheumatol. 39, 2294–2302 (2012).
De Lauretis, A. et al. Serum interleukin 6 is predictive of early functional decline and mortality in interstitial lung disease associated with systemic sclerosis. J. Rheumatol. 40, 435–446 (2013).
Cottin, V., Significance of connective tissue disease features in pulmonary fibrosis. Eur. Resp. Rev. 22, 273–280 (2013).
Tsuchiya, Y. et al. Lung diseases directly associated with rheumatoid arthritis and their relationship to outcome. Eur. Respir. J. 37, 1411–1417 (2011).
Szodoray, P. et al. Distinct phenotypes in mixed connective tissue disease: subgroups and survival. Lupus 21, 1412–1422 (2012).
Kim, E. J. et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur. Respir. J. 35, 1322–1328 (2010).
Fischer, A., West, S. G., Swigris, J. J., Brown, K. K. & du Bois, R. M. Connective tissue disease-associated interstitial lung disease: a call for clarification. Chest 138, 251–256 (2010).
Mira-Avendano, I. C. et al. A retrospective review of clinical features and treatment outcomes in steroid-resistant interstitial lung disease from polymyositis/ dermatomyositis. Respir. Med. 107, 890–896 (2013).
Gunnarsson, R. et al. Prevalence and severity of interstitial lung disease in mixed connective tissue disease: a nationwide, cross-sectional study. Ann. Rheum. Dis. 71, 1966–1972 (2012).
Kobayashi, H. et al. Clinicopathological features of pure mica pneumoconiosis associated with Sjögren syndrome. Am. J. Ind. Med. 45, 246–250 (2004).
Yamadori, I. et al. Nonspecific interstitial pneumonia as pulmonary involvement of primary Sjögren's syndrome. Rheumatol. Int. 22, 89–92 (2002).
Travis, W. D. et al. An official American Thoracic Society/ European Respiratory Society statement: update on the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 188, 733–748 (2013).
Tansey, D. et al. Variations in histological patterns of interstitial pneumonia between connective tissue disorders and their relationship to prognosis. Histopathology 44, 585–596 (2004).
Bjoraker, J. A. et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 157, 199–203 (1998).
Bouros, D. et al. Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am. J. Respir. Crit. Care Med. 165, 1581–1586 (2002).
Douglas, W. W. et al. Polymyositis-dermatomyositis-associated interstitial lung disease. Am. J. Respir. Crit. Care Med. 164, 1182–1185 (2001).
Ito, I. et al. Pulmonary manifestations of primary Sjögren's syndrome: a clinical, radiologic, and pathologic study. Am. J. Respir. Crit. Care Med. 171, 632–638 (2005).
Fischer, A. et al. Clinically significant interstitial lung disease in limited scleroderma: histopathology, clinical features, and survival. Chest 134, 601–605 (2008).
Nakamura, Y. et al. Rheumatoid lung disease: prognostic analysis of 54 biopsy-proven cases. Respir. Med. 106, 1164–1169 (2012).
Song, J. W. et al. Pathologic and radiologic differences between idiopathic and collagen vascular disease-related usual interstitial pneumonia. Chest 136, 23–30 (2009).
Flaherty, K. R. et al. Fibroblastic foci in usual interstitial pneumonia: idiopathic versus collagen vascular disease. Am. J. Respir. Crit. Care Med. 15, 1410–1415 (2003).
Park, J. H. et al. Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease-related subtypes. Am. J. Respir. Crit. Care Med. 175, 705–711 (2007).
Assayag, D. et al. Rheumatoid-arthritis-associated interstitial lung disease: radiological identification of usual interstitial pneumonia pattern. Radiology 270, 583–588 (2014).
Strange, C. & Highland, K. B. Interstitial lung disease in the patient who has connective tissue disease. Clin. Chest Med. 25, 549–559 (2004).
Mittoo, S. et al. Ascertainment of collagen vascular disease in patients presenting with interstitial lung disease. Respir. Med. 103, 1152–1158 (2009).
Romagnoli, M. et al. Idiopathic nonspecific interstitial pneumonia: an interstitial lung disease associated with autoimmune disorders? Eur. Respir. J. 38, 384–391 (2011).
Kinder, B. W. et al. Idiopathic nonspecific interstitial pneumonia: lung manifestation of undifferentiated connective tissue disease? Am. J. Respir. Crit. Care Med. 176, 691–697 (2007).
Mosca, M., Neri, R. & Bombardieri, S. Undifferentiated connective tissue diseases (UCTD): a review of the literature and a proposal for preliminary classification criteria. Clin. Exp. Rheumatol. 17, 15–20 (1999).
Corte, T. J. et al. Significance of connective tissue disease features in idiopathic interstitial pneumonia. Eur. Respir. J. 39, 661–668 (2012).
Fischer, A. et al. Anti-th/to positivity in a cohort of patients with idiopathic pulmonary fibrosis. J. Rheumatol. 33, 1600–1605 (2006).
Vij, R., Noth, I. & Strek, M. E. Autoimmune-featured interstitial lung disease: a distinct entity. Chest 140, 1292–1299 (2011).
Collard, H. R. et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 176, 636–643 (2007).
Parambil, J. G., Myers, J. L., Aubry, M. C. & Ryu, J. H. Causes and prognosis of diffuse alveolar damage diagnosed on surgical lung biopsy. Chest 132, 50–57 (2007).
Park, I. N. et al. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest 132, 214–220 (2007).
Suda, T. et al. Acute exacerbation of interstitial pneumonia associated with collagen vascular diseases. Respir. Med. 103, 846–853 (2009).
Tachikawa, R. et al. Clinical features and outcome of acute exacerbation of interstitial pneumonia: collagen vascular diseases-related versus idiopathic. Respiration 83, 20–27 (2012).
Wells, A. U. Pulmonary function tests in connective tissue disease. Semin. Respir. Crit. Care Med. 28, 379–388 (2007).
Franquet, T., Giménez, A., Monill, J. M., Díaz, C. & Geli, C. Primary Sjögren's syndrome and associated lung disease: CT findings in 50 patients. AJR Am. J. Roentgenol. 169, 655–658 (1997).
Fenlon, H. M., Doran, M., Sant, S. M. & Breatnach, E. High-resolution chest CT in systemic lupus erythematosus. AJR Am. J. Roentgenol. 166, 301–307 (1996).
Dawson, J. K., Fewins, H. E., Desmond, J., Lynch, M. P. & Graham, D. R. Fibrosing alveolitis in patients with rheumatoid arthritis as assessed by high resolution computed tomography, chest radiography, and pulmonary function tests. Thorax 56, 622–627 (2001).
Hyland, R. H. et al. A systematic controlled study of pulmonary abnormalities in rheumatoid arthritis. J. Rheumatol. 10, 395–405 (1983).
Jurik, A. G., Davidsen, D. & Graudal, H. Prevalence of pulmonary involvement in rheumatoid arthritis and its relationship to some characteristics of the patients. A radiological and clinical study. Scand. J. Rheumatol. 11, 217–224 (1982).
Goh, N. S. et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am. J. Respir. Crit. Care Med. 177, 1248–1254 (2008).
Coghlan, J. G. et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann. Rheum. Dis. 73, 1340–1349 (2014).
Cottin, V. et al. Combined pulmonary fibrosis and emphysema syndrome in connective tissue disease. Arthritis Rheum. 63, 295–304 (2011).
Cottin, V. & Cordier, J. F. Combined pulmonary fibrosis and emphysema in connective tissue disease. Curr. Opin. Pulm. Med. 18, 418–427 (2012).
Steen, V. D., Conte, C., Owens, G. R. & Medsger T. A. Jr. Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum. 37, 1283–1289 (1994).
Steen V. & Medsger T. A. Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 43, 2437–2444 (2000).
Zappala, C. J. et al. Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur. Respir. J. 35, 830–836 (2010).
Richeldi, L. et al. Relative versus absolute change in forced vital capacity in idiopathic pulmonary fibrosis. Thorax 67, 407–411 (2012).
Moore, O. A. et al. Extent of disease on high-resolution computed tomography lung is a predictor of decline and mortality in systemic sclerosis-related interstitial lung disease. Rheumatology (Oxford) 52, 155–160 (2013).
Tashkin, D. P. et al. Cyclophosphamide versus placebo in scleroderma lung disease. N. Engl. J. Med. 354, 2655–2666 (2006).
Hasegawa, M. et al. Use of serum clara cell 16-kDa (CC16) levels as a potential indicator of active pulmonary fibrosis in systemic sclerosis. J. Rheumatol. 38, 877–884 (2011).
Bonella, F. et al. Surfactant protein D and KL-6 serum levels in systemic sclerosis: correlation with lung and systemic involvement. Sarcoidosis Vasc. Diffuse Lung Dis. 28, 27–33 (2011).
Goh, N. S. et al. Bronchoalveolar lavage cellular profiles in patients with systemic sclerosis-associated interstitial lung disease are not predictive of disease progression. Arthritis Rheum. 56, 2005–2012 (2007).
Strange, C. et al. Bronchoalveolar lavage and response to cyclophosphamide in scleroderma interstitial lung disease. Am. J. Respir. Crit. Care Med. 177, 91–98 (2008).
Hoyles, R. K. et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum. 54, 3962–3970 (2006).
Seibold, J. R. et al. Randomized, prospective, placebo-controlled trial of bosentan in interstitial lung disease secondary to systemic sclerosis. Arthritis Rheum. 62, 2101–2108 (2010).
Kowal-Bielecka, O. et al. EULAR recommendations for the treatment of systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group (EUSTAR). Ann. Rheum. Dis. 68, 620–628 (2009).
Tashkin, D. P. et al. Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. Am. J. Respir. Crit. Care Med. 176, 1026–1034 (2007).
Fischer, A. et al. Mycophenolate mofetil improves lung function in connective tissue disease-associated interstitial lung disease. J. Rheumatol. 40, 640–646 (2013).
White, B., Moore, W. C., Wigley, F. M., Xiao, H. Q. & Wise, R. A. Cyclophosphamide is associated with pulmonary function and survival benefit in patients with scleroderma and alveolitis. Ann. Intern. Med. 132, 947–954 (2000).
Steen V. D. & Medsger T. A. Jr. Case–control study of corticosteroids and other drugs that could precipitate or protect from the development of scleroderma renal crisis. Arthritis Rheum. 41, 1613–1619 (1998).
DeMarco, P. J. et al. Predictors and outcomes of scleroderma renal crisis: the high-dose versus low-dose D-penicillamine in early diffuse systemic sclerosis trial. Arthritis Rheum. 46, 2983–2989 (2002).
Nawata, Y. et al. Corticosteroid resistant interstitial pneumonitis in dermatomyositis/ polymyositis: prediction and treatment with cyclosporine. J. Rheumatol. 26, 1527–1533 (1999).
Marie, I. et al. Interstitial lung disease in polymyositis and dermatomyositis. Arthritis Rheum. 47, 614–622 (2002).
Raghu, G. et al. Prednisolone, azathioprine and N-acetylcysteine for pulmonary fibrosis. N. Engl. J. Med. 366, 1968–1977 (2012).
Demedts, M. et al. High dose acetylcysteine in idiopathic pulmonary fibrosis. N. Engl. J. Med. 353, 2229–2242 (2005).
Noble, P. W. et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 377, 1760–1769 (2011).
Burt, R. K. et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trial. Lancet 378, 498–506 (2011).
Nash, R. A. et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for severe systemic sclerosis: long-term follow-up of the US multicenter pilot study. Blood 110, 1388–1396 (2007).
Sem, M., Molberg, O., Lund, M. B. & Gran, J. T. Rituximab treatment of the anti-synthetase syndrome: a retrospective case series. Rheumatology 48, 968–971 (2009).
Daoussis, D. et al. Experience with rituximab in scleroderma: results from a 1-year, proof-of-principle study. Rheumatology 49, 271–280 (2010).
Daoussis, D. et al. Effect of long-term treatment with rituximab on pulmonary function and skin fibrosis in patients with diffuse systemic sclerosis. Clin. Exp. Rheumatol. 30, S17–S22 (2012).
Keir, G. J. et al. Severe interstitial lung disease in connective tissue disease: rituximab as rescue therapy. Eur. Respir. J. 40, 641–648 (2012).
Acknowledgements
The authors of this Review are supported by the NIHR Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
A.U.W. declares the following competing interests: consultancy fees from Boehringer Ingelheim, Genentech, Gilead, Intermune, MedImmune, Takeda; and honoraria from Actelion Pharmaceuticals, Beohringer Ingelheim and Intermune. C.P.D. declares that he has received consultancy fees, honoraria and research funding from Actelion Pharmaceuticals, and consultancy fees and honoraria from GlaxoSmithKline, Merck-Serono, Novartis, Pfizer and Sanofi-Aventis.
Rights and permissions
About this article
Cite this article
Wells, A., Denton, C. Interstitial lung disease in connective tissue disease—mechanisms and management. Nat Rev Rheumatol 10, 728–739 (2014). https://doi.org/10.1038/nrrheum.2014.149
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2014.149
This article is cited by
-
The usual Interstitial pneumonia pattern in autoimmune rheumatic diseases
BMC Pulmonary Medicine (2023)
-
Decreased Th1 Cells and Increased Th2 Cells in Peripheral Blood Are Associated with Idiopathic Inflammatory Myopathies Patients with Interstitial Lung Disease
Inflammation (2023)
-
Clinical and radiological features of lung disorders related to connective-tissue diseases: a pictorial essay
Insights into Imaging (2022)
-
Lung involvement in children with newly diagnosed rheumatic diseases: characteristics and associations
Pediatric Rheumatology (2022)
-
Serum MMP-9, SP-D, and VEGF levels reflect the severity of connective tissue disease-associated interstitial lung diseases
Advances in Rheumatology (2022)