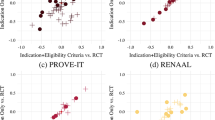
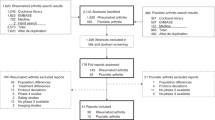
Abstract
Comparative effectiveness research (CER) is a growing area of investigation aimed at determining the most beneficial treatments for patients in view of their clinical characteristics. CER provides personalized treatment information that cannot be obtained from traditional clinical trials. However, many analytical challenges to performing CER remain, particularly in nonexperimental settings. The main obstacles to CER include bias from variation in use of treatments, and heterogeneity in types and quality of data across registries. Increasing standardization of care and consensus among stakeholders regarding CER methodology will strengthen the validity of CER from observational data. Innovations in outcomes measurement, and the ability to repurpose electronic health record data for research will increase the capability to assess treatment effects by CER in clinical practice. Investment in infrastructure, informatics, and data management to sustain high-quality registries, along with engagement of stakeholders to maintain a co-ordinated research agenda, are essential for successful CER in rheumatology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Conway, P. H. & Clancy, C. Comparative-effectiveness research—implications of the Federal Coordinating Council's report. N. Engl. J. Med. 361, 328–330 (2009).
Tunis, S., Clancy, C., Helms, W. D., McGinnis, J. M. & Pearson, S. D. Roundtable on expanding capacity for comparative effectiveness research in the United States: discussion took place on June 3, 2007, at the Academy Health Annual Research Meeting in Orlando, FL. Health Serv. Res. 44, 327–342 (2009).
Blackstone, E. A., Fuhr, J. P., Jr . & Ziernicki, D. Will comparative effectiveness research finally succeed? Biotechnol. Healthc. 9, 22–26 (2012).
Selby, J. V., Beal, A. C. & Frank, L. The Patient-Centered Outcomes Research Institute (PCORI) national priorities for research and initial research agenda. JAMA 307, 1583–1584 (2012).
Tunis, S. R., Benner, J. & McClellan, M. Comparative effectiveness research: Policy context, methods development and research infrastructure. Stat. Med. 29, 1963–1976 (2010).
Clement, F. M. et al. Using effectiveness and cost-effectiveness to make drug coverage decisions: a comparison of Britain, Australia, and Canada. JAMA 302, 1437–1443 (2009).
Curtis, J. R. et al. A comparison of patient characteristics and outcomes in selected European and U.S. rheumatoid arthritis registries. Semin. Arthritis Rheum. 40, 2–14 (2010).
Thorpe, K. E. et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J. Clin. Epidemiol. 62, 464–475 (2009).
Berry, D. A. Bayesian clinical trials. Nat. Rev. Drug Discov. 5, 27–36 (2006).
Huber, A. M., Tomlinson, G. A., Koren, G. & Feldman, B. M. Amitriptyline to relieve pain in juvenile idiopathic arthritis: a pilot study using Bayesian metaanalysis of multiple N-of-1 clinical trials. J. Rheumatol. 34, 1125–1132 (2007).
Gabler, N. B., Duan, N., Vohra, S. & Kravitz, R. L. N-of-1 trials in the medical literature: a systematic review. Med. Care 49, 761–768 (2011).
Platt, R. et al. The U.S. Food and Drug Administration's Mini-Sentinel program: status and direction. Pharmacoepidemiol. Drug Saf. 21 (Suppl. 1), 1–8 (2012).
Brown, J. S. et al. Distributed health data networks: a practical and preferred approach to multi-institutional evaluations of comparative effectiveness, safety, and quality of care. Med. Care 48, S45–S51 (2010).
Canhao, H. et al. Comparative effectiveness and predictors of response to tumour necrosis factor inhibitor therapies in rheumatoid arthritis. Rheumatology (Oxford) 51, 2020–2026 (2012).
Moots, R. J. & Naisbett-Groet, B. The efficacy of biologic agents in patients with rheumatoid arthritis and an inadequate response to tumour necrosis factor inhibitors: a systematic review. Rheumatology (Oxford) 51, 2252–2261 (2012).
Schoels, M., Aletaha, D., Smolen, J. S. & Wong, J. B. Comparative effectiveness and safety of biological treatment options after tumour necrosis factor alpha inhibitor failure in rheumatoid arthritis: systematic review and indirect pairwise meta-analysis. Ann. Rheum. Dis. 71, 1303–1308 (2012).
Zink, A. et al. Effectiveness of tumor necrosis factor inhibitors in rheumatoid arthritis in an observational cohort study: comparison of patients according to their eligibility for major randomized clinical trials. Arthritis Rheum. 54, 3399–3407 (2006).
Haynes, K. et al. Tumor necrosis factor alpha inhibitor therapy and cancer risk in chronic immune-mediated diseases. Arthritis Rheum. 65, 48–58 (2013).
Aletaha, D. et al. Reporting disease activity in clinical trials of patients with rheumatoid arthritis: EULAR/ACR collaborative recommendations. Arthritis Rheum. 59, 1371–1377 (2008).
Bombardier, C. et al. Canadian Rheumatology Association recommendations for the pharmacological management of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs: part II safety. J. Rheumatol. 39, 1583–1602 (2012).
Bykerk, V. P. et al. Canadian Rheumatology Association recommendations for pharmacological management of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs. J. Rheumatol. 39, 1559–1582 (2012).
Singh, J. A. & Cameron, D. R. Summary of AHRQ's comparative effectiveness review of drug therapy for rheumatoid arthritis (RA) in adults—an update. J. Manag. Care Pharm. 18, S1–S18 (2012).
Singh, J. A. et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res. (Hoboken) 64, 625–639 (2012).
Bernatsky, S., Lix, L., O'Donnell, S. & Lacaille, D. Consensus statements for the use of administrative health data in rheumatic disease research and surveillance. J. Rheumatol. 24, 66–73 (2013).
Suissa, S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol. Drug Saf. 16, 241–249 (2007).
Dixon, W. G. et al. EULAR points to consider when establishing, analysing and reporting safety data of biologics registers in rheumatology. Ann. Rheum. Dis. 69, 1596–1602 (2010).
Pincus, T., Brooks, R. H. & Callahan, L. F. A proposed 30–45 minute 4 page standard protocol to evaluate rheumatoid arthritis (SPERA) that includes measures of inflammatory activity, joint damage, and longterm outcomes. J. Rheumatol. 26, 473–480 (1999).
Pincus, T., Swearingen, C. J., Bergman, M. & Yazici, Y. RAPID3 (Routine Assessment of Patient Index Data 3), a rheumatoid arthritis index without formal joint counts for routine care: proposed severity categories compared to disease activity score and clinical disease activity index categories. J. Rheumatol. 35, 2136–2147 (2008).
Neogi, T. et al. The 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for rheumatoid arthritis: Phase 2 methodological report. Arthritis Rheum. 62, 2582–2591 (2010).
Lopez-Olivo, M. A., Kallen, M. A., Ortiz, Z., Skidmore, B. & Suarez-Almazor, M. E. Quality appraisal of clinical practice guidelines and consensus statements on the use of biologic agents in rheumatoid arthritis: a systematic review. Arthritis Rheum. 59, 1625–1638 (2008).
Davis, D. A., Thomson, M. A., Oxman, A. D. & Haynes, R. B. Changing physician performance. A systematic review of the effect of continuing medical education strategies. JAMA 274, 700–705 (1995).
DeWitt, E. M. et al. Consensus treatment plans for new-onset systemic juvenile idiopathic arthritis. Arthritis Care Res. (Hoboken) 64, 1001–1010 (2012).
Mina, R. et al. Consensus treatment plans for induction therapy of newly diagnosed proliferative lupus nephritis in juvenile systemic lupus erythematosus. Arthritis Care Res. (Hoboken) 64, 375–383 (2012).
Khanna, D. et al. Patient-Reported Outcomes Measurement Information System (PROMIS®)—The future of measuring patient reported outcomes in rheumatology. Arthritis Care Res. (Hoboken) 63, S486–S490 (2011).
Acknowledgements
E. M. DeWitt receives grant support for research in the Pediatric Rheumatology Care and Outcomes Improvement Network.
Author information
Authors and Affiliations
Contributions
E. M. DeWitt and H. I. Brunner contributed substantially to researching data for the article, discussion of content, writing the article and to reviewing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
DeWitt, E., Brunner, H. The landscape of comparative effectiveness research in rheumatology. Nat Rev Rheumatol 10, 57–62 (2014). https://doi.org/10.1038/nrrheum.2013.140
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2013.140
This article is cited by
-
Development of practice and consensus-based strategies including a treat-to-target approach for the management of moderate and severe juvenile dermatomyositis in Germany and Austria
Pediatric Rheumatology (2018)
-
Optimizing treatment in paediatric rheumatology—lessons from oncology
Nature Reviews Rheumatology (2015)