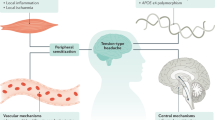
Abstract
According to the revised 2nd Edition of the International Classification of Headache Disorders, primary headaches can be categorized as chronic or episodic; chronic migraine is defined as headaches in the absence of medication overuse, occurring on ≥15 days per month for ≥3 months, of which headaches on ≥8 days must fulfill the criteria for migraine without aura. Prevalence and incidence data for chronic migraine are still uncertain, owing to the heterogeneous definitions used to identify the condition in population-based studies over the past two decades. Chronic migraine is severely disabling and difficult to manage, as affected patients experience substantially more-frequent headaches, comorbid pain and affective disorders, and fewer pain-free intervals, than do those with episodic migraine. Data on the treatment of chronic migraine are scarce because most migraine-prevention trials excluded patients who had headaches for ≥15 days per month. Despite this lack of reliable data, a wealth of expert opinion and a few evidence-based treatment options are available for managing chronic migraine. Trial data are available for topiramate and botulinum toxin type A, and expert opinion suggests that conventional preventive therapy for episodic migraine may also be useful. This Review discusses the evolution of our understanding of chronic migraine, including its epidemiology, pathophysiology, clinical characteristics and treatment options.
Key Points
-
Chronic migraine is defined as headache on ≥15 days per month for ≥3 months; headaches on ≥8 days per month must fulfill criteria for migraine without aura
-
Treatment requires a multimodal and multidisciplinary approach, including education, behavioral therapy, regular exercise and preventive drug therapy
-
Topiramate and botulinum toxin type A have shown modest but significant efficacy in placebo-controlled trials; other preventive drugs have not been adequately studied for use in chronic migraine
-
Chronic migraine can occur with or without medication overuse; patients with medication overuse should receive advice and support on discontinuation, as well as multidisciplinary treatment for chronic migraine
-
The full therapeutic armamentarium for chronic migraine is best offered in headache referral centers
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
D'Amico, D. et al. Quality of life and disability in primary chronic daily headaches. Neurol. Sci. 24 (Suppl. 2), S97–S100 (2003).
Dodick, D. W. Clinical practice. Chronic daily headache. N. Engl. J. Med. 354, 158–165 (2006).
Wiendels, N. J. et al. Chronic frequent headache in the general population: comorbidity and quality of life. Cephalalgia 26, 1443–1450 (2006).
Monzón, M. J. & Láinez, M. J. Quality of life in migraine and chronic daily headache patients. Cephalalgia 18, 638–643 (1998).
Meletiche, D. M., Lofland, J. H. & Young, W. B. Quality-of-life differences between patients with episodic and transformed migraine. Headache 41, 573–578 (2001).
Wang, S. J., Fuh, J. L., Lu, S. R. & Juang, K. D. Outcomes and predictors of chronic daily headache in adolescents: a 2-year longitudinal study. Neurology 68, 591–596 (2007).
Guitera, V., Muñoz, P., Castillo, J. & Pascual, J. Quality of life in chronic daily headache: a study in a general population. Neurology 58, 1062–1065 (2002).
D'Amico, D. et al. Disability pattern in chronic migraine with medication overuse: a comparison with migraine without aura. Headache 45, 553–560 (2005).
Lipton, R. B. et al. Migraine headache disability and health-related quality-of-life: a population-based case–control study from England. Cephalalgia 23, 441–450 (2003).
Bigal, M. E., Serrano, D., Reed, M. & Lipton, R. B. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology 71, 559–566 (2008).
Munakata, J. et al. Economic burden of transformed migraine: results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache 49, 498–508 (2009).
Mathew, N. T., Stubits, E. & Nigam, M. Transformation of episodic migraine into daily headache: analysis of factors. Headache 22, 66–68 (1982).
Saper, J. R. in Drug-Induced Headache 1st edn (eds Diener, H. C. & Wilkinson, M.) 5–8 (Springer–Verlag Berlin and Heidelberg, New York, 1988).
Gowers, W. R. A Manual of Diseases of the Nervous System (P. Blakiston, Son & Co., Philadelphia, 1888).
Sjaastad, O. “Chronic daily headache” (“cefalea cronica quotidiana”). Cephalalgia 5 (Suppl. 2), 191–193 (1985).
Mathew, N. T. Drug induced refractory headache clinical features and management [abstract 52]. Headache 27, 305–306 (1987).
[No authors listed]. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache Classification Committee of the International Headache Society. Cephalalgia 8 (Suppl. 7), 1–96 (1988).
Stovner, L. J., Zwart, J. A., Hagen, K., Terwindt, G. M. & Pascual, J. Epidemiology of headache in Europe. Eur. J. Neurol. 13, 333–345 (2006).
Stovner, L. J. et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia 27, 193–210 (2007).
Headache Classification Committee et al. New appendix criteria open for a broader concept of chronic migraine. Cephalalgia 26, 742–746 (2006).
Manzoni, G. C. et al. Chronic migraine classification: current knowledge and future perspectives. J. Headache Pain 12, 585–592 (2011).
Saper, J. R. The mixed headache syndrome: a new perspective. Headache 22, 284–286 (1982).
Ad Hoc Committee on Classification of Headache. Classification of headache. JAMA 179, 717–718 (1962).
Silberstein, S. D. & Lipton, R. B. in Headache Ch. 12 (eds Goadsby, P. J. & Silberstein, S. D.) 201–225 (Butterworth-Heinemann, Boston, 1997).
Silberstein, S. D., Lipton, R. B., Solomon, S. & Mathew, N. T. Classification of daily and near-daily headaches: proposed revisions to the IHS criteria. Headache 34, 1–7 (1994).
Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 24 (Suppl. 1), 9–160 (2004).
Yoon, M. S., Obermann, M., Dommes, P., Diener, H. C. & Katsarava, Z. Prevalence of migraine in a population based sample in Germany: results of the GHC study [abstract PO116]. Cephalalgia 29 (1 Suppl.), 56–57 (2009).
Zeeberg, P., Olesen, J. & Jensen, R. Medication overuse headache and chronic migraine in a specialized headache centre: field-testing proposed new appendix criteria. Cephalalgia 29, 214–220 (2009).
Bigal, M., Rapoport, A., Sheftell, F., Tepper, S. & Lipton, R. The International Classification of Headache Disorders revised criteria for chronic migraine—field testing in a headache specialty clinic. Cephalalgia 27, 230–234 (2007).
Mathew, N. T. Transformed migraine. Cephalalgia 13 (Suppl. 12), 78–83 (1993).
Silberstein, S. D., Lipton, R. B. & Sliwinski, M. Classification of daily and near-daily headaches: field trial of revised IHS criteria. Neurology 47, 871–875 (1996).
Natoli, J. L. et al. Global prevalence of chronic migraine: a systematic review. Cephalalgia 30, 599–609 (2010).
Linde, M., Stovner, L. J., Zwart, J. A. & Hagen, K. Time trends in the prevalence of headache disorders. The Nord-Trondelag Health Studies (HUNT 2 and HUNT 3). Cephalalgia 31, 585–596 (2011).
Scher, A. I., Stewart, W. F., Ricci, J. A. & Lipton, R. B. Factors associated with the onset and remission of chronic daily headache in a population-based study. Pain 106, 81–89 (2003).
Bigal, M. E. & Lipton, R. B. Concepts and mechanisms of migraine chronification. Headache 48, 7–15 (2008).
Bigal, M. E. et al. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache 48, 1157–1168 (2008).
Bigal, M. E. & Lipton, R. B. Excessive opioid use and the development of chronic migraine. Pain 142, 179–182 (2009).
Bigal, M. E. & Lipton, R. B. Overuse of acute migraine medications and migraine chronification. Curr. Pain. Headache Rep. 13, 301–307 (2009).
Manack, A., Buse, D. C., Serrano, D., Turkel, C. C. & Lipton, R. B. Rates, predictors, and consequences of remission from chronic migraine to episodic migraine. Neurology 76, 711–718 (2011).
Goadsby, P. J., Charbit, A. R., Andreou, A. P., Akerman, S. & Holland, P. R. Neurobiology of migraine. Neuroscience 161, 327–341 (2009).
Burstein, R. Deconstructing migraine headache into peripheral and central sensitization. Pain 89, 107–110 (2001).
Goadsby, P. J. & Hargreaves, R. Refractory migraine and chronic migraine: pathophysiological mechanisms. Headache 48, 799–804 (2008).
Zagami, A. S. & Lambert, G. A. Stimulation of cranial vessels excites nociceptive neurones in several thalamic nuclei of the cat. Exp. Brain Res. 81, 552–566 (1990).
Shields, K. G. & Goadsby, P. J. Propranolol modulates trigeminovascular responses in thalamic ventroposteromedial nucleus: a role in migraine? Brain 128, 86–97 (2005).
Andreou, A. P., Shields, K. G. & Goadsby, P. J. GABA and valproate modulate trigeminovascular nociceptive transmission in the thalamus. Neurobiol. Dis. 37, 314–323 (2010).
Weiller, C. et al. Brain stem activation in spontaneous human migraine attacks. Nat. Med. 1, 658–660 (1995).
Afridi, S. K. et al. A positron emission tomographic study in spontaneous migraine. Arch. Neurol. 62, 1270–1275 (2005).
Bahra, A., Matharu, M. S., Buchel, C., Frackowiak, R. S. & Goadsby, P. J. Brainstem activation specific to migraine headache. Lancet 357, 1016–1017 (2001).
Matharu, M. S. et al. Central neuromodulation in chronic migraine patients with suboccipital stimulators: a PET study. Brain 127, 220–230 (2004).
Welch, K. M., Nagesh, V., Aurora, S. K. & Gelman, N. Periaqueductal grey matter dysfunction in migraine: cause or the burden of illness? Headache 41, 629–637 (2001).
Moulton, E. A. et al. Interictal dysfunction of a brainstem descending modulatory center in migraine patients. PLoS ONE 3, e3799 (2008).
Bouhassira, D., Bing, Z. & Le Bars, D. Studies of the brain structures involved in diffuse noxious inhibitory controls: the mesencephalon. J. Neurophysiol. 64, 1712–1723 (1990).
Aurora, S. K. Is chronic migraine one end of a spectrum of migraine or a separate entity? Cephalalgia 29, 597–605 (2009).
Aurora, S. K. Spectrum of illness: understanding biological patterns and relationships in chronic migraine. Neurology 72 (5 Suppl.), S8–S13 (2009).
Limmroth, V., Katsarava, Z., Fritsche, G., Przywara, S. & Diener, H. C. Features of medication overuse headache following overuse of different acute headache drugs. Neurology 59, 1011–1014 (2002).
Zeeberg, P., Olesen, J. & Jensen, R. Probable medication-overuse headache: the effect of a 2-month drug-free period. Neurology 66, 1894–1898 (2006).
De Felice, M. & Porreca, F. Opiate-induced persistent pronociceptive trigeminal neural adaptations: potential relevance to opiate-induced medication overuse headache. Cephalalgia 29, 1277–1284 (2009).
De Felice, M. et al. Triptan-induced latent sensitization: a possible basis for medication overuse headache. Ann. Neurol. 67, 325–337 (2010).
Diener, H. C., et al. Integrated headache care. Cephalalgia 31, 1039–1047 (2011).
Antonaci, F., Dumitrache, C., De Cillis, I. & Allena, M. A review of current European treatment guidelines for migraine. J. Headache Pain 11, 13–19 (2010).
British Association for the Study of Headache. Guidelines for all healthcare professionals in the diagnosis and management of migraine, tension-type headache, cluster headache and medication-overuse headache. BASH [online], (2010).
Rains, J. C. & Penzien, D. B. Behavioral treatment strategies for migraine and tension-type headache: a review of the evidence and future directions. Expert Rev. Neurother. 2, 749–760 (2002).
Gallai, V., Sarchielli, P. for the Ad Hoc Committee for the Diagnostic and Therapeutic Guidelines of Migraine and Cluster Headache. Diagnostic and therapeutic guidelines for migraine. Italian Society for the Study of Headaches (SISC). J. Headache Pain 2 (Suppl. 1), S125–S129 (2001).
Silberstein, S. D. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommitee of the American Academy of Neurology. Neurology 55, 754–762 (2000).
Evers, S. et al. EFNS guideline on the drug treatment of migraine—revised report of an EFNS task force. Eur. J. Neurol. 16, 968–981 (2009).
Yurekli, V. A. et al. The effect of sodium valproate on chronic daily headache and its subgroups. J. Headache Pain 9, 37–41 (2008).
Silvestrini, M. et al. Topiramate in the treatment of chronic migraine. Cephalalgia 23, 820–824 (2003).
Diener, H. C. et al. Topiramate reduces headache days in chronic migraine: a randomized, double-blind, placebo-controlled study. Cephalalgia 27, 814–823 (2007).
Silberstein, S. D. et al. Efficacy and safety of topiramate for the treatment of chronic migraine: a randomized, double-blind, placebo-controlled trial. Headache 47, 170–180 (2007).
Diener, H. C. et al. Utility of topiramate for the treatment of patients with chronic migraine in the presence or absence of acute medication overuse. Cephalalgia 29, 1021–1027 (2009).
Foster, L., Clapp, L., Erickson, M. & Jabbari, B. Botulinum toxin A and chronic low back pain: a randomized, double-blind study. Neurology 56, 1290–1293 (2001).
Mathew, N. T. et al. Botulinum toxin type A (BOTOX) for the prophylactic treatment of chronic daily headache: a randomized, double-blind, placebo-controlled trial. Headache 45, 293–307 (2005).
Ranoux, D., Attal, N., Morain, F. & Bouhassira, D. Botulinum toxin type A induces direct analgesic effects in chronic neuropathic pain. Ann. Neurol. 64, 274–283 (2008).
Freitag, F. G., Diamond, S., Diamond, M. & Urban, G. Botulinum toxin type A in the treatment of chronic migraine without medication overuse. Headache 48, 201–209 (2008).
Aoki, K. R. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology 26, 785–793 (2005).
Gazerani, P., Staahl, C., Drewes, A. M. & Arendt-Nielsen, L. The effects of botulinum toxin type A on capsaicin-evoked pain, flare, and secondary hyperalgesia in an experimental human model of trigeminal sensitization. Pain 122, 315–325 (2006).
Bergerot, A. et al. Animal models of migraine: looking at the component parts of a complex disorder. Eur. J. Neurosci. 24, 1517–1534 (2006).
Silberstein, S., Mathew, N., Saper, J. & Jenkins, S. Botulinum toxin type A as a migraine preventive treatment. For the BOTOX Migraine Clinical Research Group. Headache 40, 445–450 (2000).
Evers, S. et al. Botulinum toxin A in the prophylactic treatment of migraine—a randomized, double-blind, placebo-controlled study. Cephalalgia 24, 838–843 (2004).
Silberstein, S. D. et al. Botulinum toxin type A for the prophylactic treatment of chronic daily headache: a randomized, double-blind, placebo-controlled trial. Mayo Clin. Proc. 80, 1126–1137 (2005).
Aurora, S. K., Gawel, M., Brandes, J. L., Pokta, S. & Vandenburgh, A. M. Botulinum toxin type a prophylactic treatment of episodic migraine: a randomized, double-blind, placebo-controlled exploratory study. Headache 47, 486–499 (2007).
Saper, J. R., Mathew, N. T., Loder, E. W., DeGryse, R. & VanDenburgh, A. M. A double-blind, randomized, placebo-controlled comparison of botulinum toxin type a injection sites and doses in the prevention of episodic migraine. Pain Med. 8, 478–485 (2007).
Vo, A. H. et al. Botulinum toxin type-a in the prevention of migraine: a double-blind controlled trial. Aviat. Space Environ. Med. 78 (5 Suppl.), B113–B118 (2007).
Dodick, D. W. et al. Botulinum toxin type a for the prophylaxis of chronic daily headache: subgroup analysis of patients not receiving other prophylactic medications: a randomized double-blind, placebo-controlled study. Headache 45, 315–324 (2005).
Aurora, S. K. et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia 30, 793–803 (2010).
Diener, H. C. et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia 30, 804–814 (2010).
Dodick, D. W. et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache 50, 921–936 (2010).
Beran, R. G. & Spira, P. J. Levetiracetam in chronic daily headache: a double-blind, randomised placebo-controlled study. (The Australian KEPPRA Headache Trial [AUS-KHT]). Cephalalgia 31, 530–536 (2011).
Saper, J. R. et al. Occipital nerve stimulation for the treatment of intractable chronic migraine headache: ONSTIM feasibility study. Cephalalgia 31, 271–285 (2011).
Lipton, R. B. et al. PRISM study: occipital nerve stimulation for treatment-refractory migraine [abstract PO47]. Cephalalgia 29 (1 Suppl.), 30 (2009).
Nicholson, R. A., Buse, D. C., Andrasik, F. & Lipton, R. B. Nonpharmacologic treatments for migraine and tension-type headache: how to choose and when to use. Curr. Treat. Options Neurol. 13, 28–40 (2011).
Coeytaux, R. R. et al. A randomized, controlled trial of acupuncture for chronic daily headache. Headache 45, 1113–1123 (2005).
Park, J. M., Park, S. U., Jung, W. S. & Moon, S. K. Carthami-Semen acupuncture point injection for chronic daily headache: a pilot, randomised, double-blind, controlled trial. Complement. Ther. Med. 19 (Suppl. 1), S19–S25 (2011).
Calhoun, A. H. & Ford, S. Behavioral sleep modification may revert transformed migraine to episodic migraine. Headache 47, 1178–1183 (2007).
Acknowledgements
The authors thank A. O. Horner and P. Kukovich of Imprint Publication Science, for their assistance with the literature search and language editing. They also performed the systematic literature search for Allergan described in the Review criteria. H.-C. Diener acknowledges the support of the German Research Foundation (Deutsche Forschungsgemeinschaft), the Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung) and the European Union. D. W. Dodick acknowledges grant support from the National Institute of Neurological Disorders and Stroke (NINDS) and the NIH. P. J. Goadsby has consulted for, advised or collaborated with NINDS, the Netherlands Organization for Scientific Research, the Organization for Understanding Cluster Headaches, and the Organization for the Understanding of Cluster Headache (UK). R. B. Lipton has acted as a reviewer for the National Institute on Aging and NINDS. Professor Lipton acknowledges grant support from the Migraine Research Foundation, the National Headache Foundation, and the NIH (grants PO1AG03949, PO1AG027734, RO1AG025119, RO1AG022374-06A2, RO1AG034119, RO1AG12101, K23AG030857, K23NS05140901A1 and K23NS47256). S. D. Silberstein is on the advisory panel for and receives honoraria from NINDS.
Author information
Authors and Affiliations
Contributions
H.-C. Diener, D. W. Dodick and S. D. Silberstein contributed to all aspects of this Review. P. J. Goadsby and J. Olesen contributed to the discussion of content, writing and review and/or editing of the manuscript before submission. R. B. Lipton contributed to the discussion of content and review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
H.-C. Diener received honoraria for participation in clinical trials, contribution to advisory boards or oral presentations from Addex Pharmaceuticals, Allergan, Almirall, AstraZeneca, Bayer Vital, Berlin-Chemie, Boehringer Ingelheim, Bristol-Myers Squibb, Coherex Medical, CoLucid Pharmaceuticals, GlaxoSmithKline, Grünenthal, Janssen-Cilag, Johnson & Johnson, Lilly, Medtronic, Merck Sharpe & Dohme, Minster Pharmaceuticals, Novartis, Pfizer, Pierre Fabre, Roche, Sanofi-aventis, Schaper & Brümmer, Weber & Weber and 3M Medica. He also received financial support for research projects from Allergan, Almirall, AstraZeneca, Bayer Healthcare, GlaxoSmithKline, Janssen-Cilag and Pfizer. Dr Diener has no ownership interest in and does not own stocks of any pharmaceutical company.
D. W. Dodick has, within the past 3 years, served on advisory boards and consulted for Alder Biopharmaceuticals, Allergan, Arteaus Therapeutics, Atlas Laboratories & Pharmaceuticals, Autonomic Technologies, Boston Scientific, Bristol-Myers Squibb, Coherex Medical, CoLucid Pharmaceuticals, Ferring Pharmaceuticals, GlaxoSmithKline, Impax Laboratories, Lilly, MAP Pharmaceuticals, Medtronic, Merck Sharpe & Dohme, Nautilus Neurosciences, Neuralieve, NeurAxon, Neurocore, Nevro, Novartis, NuPathe, Pfizer, and Zogenix. He has also received funding for travel, speaking or editorial activities from the Annenberg Center for Health Sciences, Cambridge University Press, CogniMed, IntraMed, Lippincott Williams & Wilkins, Miller Medical Supplies, Oxford University Press, SAGE Publications and Scientiae. Dr Dodick has received research grant support from Advanced Neurostimulation Systems, Boston Scientific, Mayo Clinic, Medtronic and St Jude Medical.
P. J. Goadsby has consulted for, advised or collaborated with Advanced Bionics, Allergan, Almirall, Amgen, Autonomic Technologies, AstraZeneca, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, CoLucid, GlaxoSmithKline, Johnson & Johnson, Lilly, MAP Pharmaceuticals, Medtronic, Merck, Sharpe & Dohme, Neuralieve, NeurAxon, NeuroTherapeutics Pharma and Pfizer.
R. B. Lipton serves as a consultant or advisory board member for, or has received honoraria from, Allergan, American Headache Society, Autonomic Technologies, Boston Scientific, Bristol-Myers Squibb, CogniMed, Diamond Headache Clinic, Endo Pharmaceuticals, GlaxoSmithKline, Lilly, Merck Serono, Nautilus Neurosciences, Neuralieve, Novartis and Pfizer. He also holds stock options in Neuralieve, a company without commercial products.
J. Olesen has received grant or research support, and/or has been a consultant or scientific advisor, and/or has been on the speakers' bureau of the following companies: Bristol-Myers-Squibb, Lundbeck, Merck Serono, NeurAxon, Union Chimique Belge and Zogenix.
S. D. Silberstein is on the advisory panel of and receives honoraria from, Allergan, Amgen, Capnia, Coherex Medical, GlaxoSmithKline, Iroko Pharmaceuticals, Lilly, MAP Pharmaceuticals, Medtronic, Merck Sharpe & Dohme, Neuralieve, NuPathe, Pfizer and St Jude Medical. He is on the speakers' bureau of and receives honoraria from Allergan, Endo Pharmaceuticals, GlaxoSmithKline, Merck Sharpe & Dohme and Zogenix. Dr Silberstein also serves as a consultant for and receives honoraria from Amgen, Nautilus Neurosciences, Novartis, OptiNose and Zogenix.
Rights and permissions
About this article
Cite this article
Diener, HC., Dodick, D., Goadsby, P. et al. Chronic migraine—classification, characteristics and treatment. Nat Rev Neurol 8, 162–171 (2012). https://doi.org/10.1038/nrneurol.2012.13
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2012.13
This article is cited by
-
Monoclonal antibodies against CGRP (R): non-responders and switchers: real world data from an austrian case series
BMC Neurology (2023)
-
Migraine
Nature Reviews Disease Primers (2022)
-
Botulinum toxin injection in the management of chronic migraine: the Saudi experience with a proposal for a new protocol
Acta Neurologica Belgica (2021)
-
Diagnosis and management of migraine in ten steps
Nature Reviews Neurology (2021)
-
The effect of greater occipital nerve blockade on the quality of life, disability and comorbid depression, anxiety, and sleep disturbance in patients with chronic migraine
Neurological Sciences (2020)