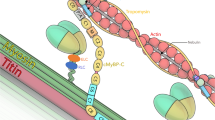
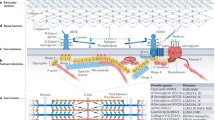
Abstract
The collagen VI-related myopathy known as Ullrich congenital muscular dystrophy is an early-onset disease that combines substantial muscle weakness with striking joint laxity and progressive contractures. Patients might learn to walk in early childhood; however, this ability is subsequently lost, concomitant with the development of frequent nocturnal respiratory failure. Patients with intermediate phenotypes of collagen VI-related myopathy display a lesser degree of weakness and a longer period of ambulation than do individuals with Ullrich congenital muscular dystrophy, and the spectrum of disease finally encompasses mild Bethlem myopathy, in which ambulation persists into adulthood. Dominant and recessive autosomal mutations in the three major collagen VI genes—COL6A1, COL6A2, and COL6A3—can underlie this entire clinical spectrum, and result in deficient or dysfunctional microfibrillar collagen VI in the extracellular matrix of muscle and other connective tissues, such as skin and tendons. The potential effects on muscle include progressive dystrophic changes, fibrosis and evidence for increased apoptosis, which potentially open avenues for pharmacological intervention. Optimized respiratory management, including noninvasive nocturnal ventilation together with careful orthopedic management, are the current mainstays of treatment and have already led to a considerable improvement in life expectancy for children with Ullrich congenital muscular dystrophy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Okada, M. et al. Primary collagen VI deficiency is the second most common congenital muscular dystrophy in Japan. Neurology 69, 1035–1042 (2007).
Norwood, F. L. et al. Prevalence of genetic muscle disease in northern England: in-depth analysis of a muscle clinic population. Brain 132, 3175–3186 (2009).
Peat, R. A. et al. Diagnosis and etiology of congenital muscular dystrophy. Neurology 71, 312–321 (2008).
Ullrich, O. Congenital, atonic–sclerotic muscular dystrophy, an additional type of heredo-degenerative illness of the neuromuscular system [German]. Z. Ges. Neurol. Psychiat. 126, 171–201 (1930).
Ullrich, O. Congenital atonic–sclerotic muscular dystrophy [German]. Monatsschr. Kinderheilkd. 47, 502–510 (1930).
Furukawa, T. & Toyokura, Y. Congenital, hypotonic–sclerotic muscular dystrophy. J. Med. Genet. 14, 426–429 (1977).
Nonaka, I. et al. A clinical and histological study of Ullrich's disease (congenital atonic-sclerotic muscular dystrophy). Neuropediatrics 12, 197–208 (1981).
Voit, T. Congenital muscular dystrophies: 1997 update. Brain Dev. 20, 65–74 (1998).
Bertini, E. & Pepe, G. Collagen type VI and related disorders: Bethlem myopathy and Ullrich scleroatonic muscular dystrophy. Eur. J. Paediatr. Neurol. 6, 193–198 (2002).
Lampe, A. K. & Bushby, K. M. Collagen VI related muscle disorders. J. Med. Genet. 42, 673–685 (2005).
Nadeau, A. et al. Natural history of Ullrich congenital muscular dystrophy. Neurology 73, 25–31 (2009).
Brinas, L. et al. Early onset collagen VI myopathies: genetic and clinical correlations. Ann. Neurol. 68, 511–520 (2010).
Jöbsis, G. J., Boers, J. M., Barth, P. G. & de Visser, M. Bethlem myopathy: a slowly progressive congenital muscular dystrophy with contractures. Brain 122, 649–655 (1999).
Scacheri, P. C. et al. Novel mutations in collagen VI genes: expansion of the Bethlem myopathy phenotype. Neurology 58, 593–602 (2002).
Bradley, W. G., Hudgson, P., Gardner-Medwin, D. & Walton, J. N. The syndrome of myosclerosis. J. Neurol. Neurosurg. Psychiatry 36, 651–660 (1973).
Merlini, L. et al. Autosomal recessive myosclerosis myopathy is a collagen VI disorder. Neurology 71, 1245–1253 (2008).
Voermans, N. C. et al. Clinical and molecular overlap between myopathies and inherited connective tissue diseases. Neuromuscul. Disord. 18, 843–856 (2008).
Alexopoulos, L. G., Youn, I., Bonaldo, P. & Guilak, F. Developmental and osteoarthritic changes in Col6a1-knockout mice: biomechanics of type VI collagen in the cartilage pericellular matrix. Arthritis Rheum. 60, 771–779 (2009).
Kirschner, J. et al. Ullrich congenital muscular dystrophy: connective tissue abnormalities in the skin support overlap with Ehlers–Danlos syndromes. Am. J. Med. Genet. A 132A, 296–301 (2005).
Voermans, N. C., Bönnemann, C. G., Lammens, M., van Engelen, B. G. & Hamel, B. C. Myopathy and polyneuropathy in an adolescent with the kyphoscoliotic type of Ehlers–Danlos syndrome. Am. J. Med. Genet. A 149A, 2311–2316 (2009).
Voermans, N. C. et al. Ehlers–Danlos syndrome due to tenascin-X deficiency: muscle weakness and contractures support overlap with collagen VI myopathies. Am. J. Med. Genet A 143A, 2215–2219 (2007).
Minamitani, T. et al. Modulation of collagen fibrillogenesis by tenascin-X and type VI collagen. Exp. Cell Res. 298, 305–315 (2004).
Sipila, L. et al. Secretion and assembly of type IV and VI collagens depend on glycosylation of hydroxylysines. J. Biol. Chem. 282, 33381–33388 (2007).
Quijano-Roy, S. et al. De novo LMNA mutations cause a new form of congenital muscular dystrophy. Ann. Neurol. 64, 177–186 (2008).
Ferreiro, A. et al. A recessive form of central core disease, transiently presenting as multi-minicore disease, is associated with a homozygous mutation in the ryanodine receptor type 1 gene. Ann. Neurol. 51, 750–759 (2002).
Jungbluth, H. et al. Autosomal recessive inheritance of RYR1 mutations in a congenital myopathy with cores. Neurology 59, 284–287 (2002).
Voermans, N. C., Bönnemann, C. G., Hamel, B. C., Jungbluth, H. & van Engelen, B. G. Joint hypermobility as a distinctive feature in the differential diagnosis of myopathies. J. Neurol. 256, 13–27 (2009).
Schessl, J. et al. Predominant fiber atrophy and fiber type disproportion in early ullrich disease. Muscle Nerve 38, 1184–1191 (2008).
Ishikawa, H. et al. Ullrich disease: collagen VI deficiency: EM suggests a new basis for muscular weakness. Neurology 59, 920–923 (2002).
Pan, T. C. et al. New molecular mechanism for Ullrich congenital muscular dystrophy: a heterozygous in-frame deletion in the COL6A1 gene causes a severe phenotype. Am. J. Hum. Genet. 73, 355–369 (2003).
Ishikawa, H. et al. Ullrich disease due to deficiency of collagen VI in the sarcolemma. Neurology 62, 620–623 (2004).
Baker, N. L. et al. Dominant collagen VI mutations are a common cause of Ullrich congenital muscular dystrophy. Hum. Mol. Genet. 14, 279–293 (2005).
Hicks, D. et al. A refined diagnostic algorithm for Bethlem myopathy. Neurology 70, 1192–1199 (2008).
Jimenez-Mallebrera, C. et al. A comparative analysis of collagen VI production in muscle, skin and fibroblasts from 14 Ullrich congenital muscular dystrophy patients with dominant and recessive COL6A mutations. Neuromuscul. Disord. 16, 571–582 (2006).
Deconinck, N. et al. Differentiating Emery–Dreifuss muscular dystrophy and collagen VI-related myopathies using a specific CT scanner pattern. Neuromuscul. Disord. 20, 517–523 (2010).
Mercuri, E. et al. Muscle magnetic resonance imaging involvement in muscular dystrophies with rigidity of the spine. Ann. Neurol. 67, 201–208 (2010).
Mercuri, E. et al. Muscle MRI in Ullrich congenital muscular dystrophy and Bethlem myopathy. Neuromuscul. Disord. 15, 303–310 (2005).
Bönnemann, C. G., Brockmann, K. & Hanefeld, F. Muscle ultrasound in Bethlem myopathy. Neuropediatrics 34, 335–336 (2003).
Keene, D. R., Engvall, E. & Glanville, R. W. Ultrastructure of type VI collagen in human skin and cartilage suggests an anchoring function for this filamentous network. J. Cell Biol. 107, 1995–2006 (1988).
Kuo, H. J., Maslen, C. L., Keene, D. R. & Glanville, R. W. Type VI collagen anchors endothelial basement membranes by interacting with type IV collagen. J. Biol. Chem. 272, 26522–26529 (1997).
Ritty, T. M., Roth, R. & Heuser, J. E. Tendon cell array isolation reveals a previously unknown fibrillin-2-containing macromolecular assembly. Structure 11, 1179–1188 (2003).
Timpl, R. & Chu, M. L. in Extracellular Matrix Assembly and Structure ( eds Yurchenco, P. D. et al.) 207–242 (Academic Press, Orlando, 1994).
Fitzgerald, J., Rich, C., Zhou, F. H. & Hansen, U. Three novel collagen VI chains, α4(VI), α5(VI), and α6(VI). J. Biol. Chem. 283, 20170–20180 (2008).
Gara, S. K. et al. Three novel collagen VI chains with high homology to the α3 chain. J. Biol. Chem. 283, 10658–10670 (2008).
Sabatelli, P. et al. Expression of the collagen VI α5 and α6 chains in normal human skin and in skin of patients with collagen VI-related myopathies. J. Invest. Dermatol. 131, 99–107 (2011).
Chu, M. L. et al. The structure of type VI collagen. Ann. N. Y Acad. Sci. 580, 55–63 (1990).
Aigner, T., Hambach, L., Söder, S., Schlötzer-Schrehardt, U. & Pöschl, E. The C5 domain of Col6A3 is cleaved off from the Col6 fibrils immediately after secretion. Biochem. Biophys. Res. Commun. 290, 743–748 (2002).
Engvall, E., Hessle, H. & Klier, G. Molecular assembly, secretion, and matrix deposition of type VI collagen. J. Cell Biol. 102, 703–710 (1986).
Furthmayr, H., Wiedemann, H., Timpl, R., Odermatt, E. & Engel, J. Electron-microscopical approach to a structural model of intima collagen. Biochem. J. 211, 303–311 (1983).
Chu, M. L. et al. Amino acid sequence of the triple-helical domain of human collagen type VI. J. Biol. Chem. 263, 18601–18606 (1988).
Colombatti, A. Mucignat, M. T. & Bonaldo, P. Secretion and matrix assembly of recombinant type VI collagen. J. Biol. Chem. 270, 13105–13111 (1995).
Bonaldo, P., Russo, V., Bucciotti, F., Doliana, R. & Colombatti, A. Structural and functional features of the α3 chain indicate a bridging role for chicken collagen VI in connective tissues. Biochemistry 29, 1245–1254 (1990).
Lamandé, S. R. et al. The role of the α3(VI) chain in collagen VI assembly. Expression of an α3(VI) chain lacking N-terminal modules N10-N7 restores collagen VI assembly, secretion, and matrix deposition in an α3(VI)-deficient cell line. J. Biol. Chem. 273, 7423–7430 (1998).
Baldock, C., Sherratt, M. J., Shuttleworth, C. A. & Kielty, C. M. The supramolecular organization of collagen VI microfibrils. J. Mol. Biol. 330, 297–307 (2003).
Wiberg, C. et al. Biglycan and decorin bind close to the N-terminal region of the collagen VI triple helix. J. Biol. Chem. 276, 18947–18952 (2001).
Bowe, M. A., Mendis, D. B. & Fallon, J. R. The small leucine-rich repeat proteoglycan biglycan binds to α-dystroglycan and is upregulated in dystrophic muscle. J. Cell Biol. 148, 801–810 (2000).
Rafii, M. S. et al. Biglycan binds to α- and γ-sarcoglycan and regulates their expression during development. J. Cell Physiol. 209, 439–447 (2006).
Aumailley, M., Specks, U. & Timpl, R. Cell adhesion to type VI collagen. Biochem. Soc. Trans. 19, 843–847 (1991).
Klein, G., Müller, C. A., Tillet, E., Chu, M. L. & Timpl, R. Collagen type VI in the human bone marrow microenvironment: a strong cytoadhesive component. Blood 86, 1740–1748 (1995).
Kawahara, G. et al. Reduced cell anchorage may cause sarcolemma-specific collagen VI deficiency in Ullrich disease. Neurology 69, 1043–1049 (2007).
Atkinson, J. C., Rühl, M., Becker, J., Ackermann, R. & Schuppan, D. Collagen VI regulates normal and transformed mesenchymal cell proliferation in vitro. Exp. Cell Res. 228, 283–291 (1996).
Perris, R., Kuo, H. J., Glanville, R. W. & Bronner-Fraser, M. Collagen type VI in neural crest development: distribution in situ and interaction with cells in vitro. Dev. Dyn. 198, 135–49 (1993).
Iyengar, P. et al. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J. Clin. Invest. 115, 1163–1176 (2005).
Rühl, M. et al. Soluble collagen VI drives serum-starved fibroblasts through S phase and prevents apoptosis via down-regulation of Bax. J. Biol. Chem. 274, 34361–34368 (1999).
Irwin, W. A. et al. Mitochondrial dysfunction and apoptosis in myopathic mice with collagen VI deficiency. Nat. Genet. 35, 367–371 (2003).
Nakajima, I., Muroya, S., Tanabe, R. & Chikuni, K. Extracellular matrix development during differentiation into adipocytes with a unique increase in type V and VI collagen. Biol. Cell 94, 197–203 (2002).
Zou, Y., Zhang, R. Z., Sabatelli, P., Chu, M. L. & Bönnemann, C. G. Muscle interstitial fibroblasts are the main source of collagen VI synthesis in skeletal muscle: implications for congenital muscular dystrophy types Ullrich and Bethlem. J. Neuropathol. Exp. Neurol. 67, 144–154 (2008).
Braghetta, P. et al. An enhancer required for transcription of the Col6a1 gene in muscle connective tissue is induced by signals released from muscle cells. Exp. Cell Res. 314, 3508–3518 (2008).
Bonaldo, P. et al. Collagen VI deficiency induces early onset myopathy in the mouse: an animal model for Bethlem myopathy. Hum. Mol. Genet. 7, 2135–2140 (1998).
Angelin, A. et al. Mitochondrial dysfunction in the pathogenesis of Ullrich congenital muscular dystrophy and prospective therapy with cyclosporins. Proc. Natl Acad. Sci. USA 104, 991–996 (2007).
Angelin, A., Bonaldo, P. & Bernardi, P. Altered threshold of the mitochondrial permeability transition pore in Ullrich congenital muscular dystrophy. Biochim. Biophys. Acta 1777, 893–896 (2008).
Tiepolo, T. et al. The cyclophilin inhibitor Debio 025 normalizes mitochondrial function, muscle apoptosis and ultrastructural defects in Col6a1−/− myopathic mice. Br. J. Pharmacol. 157, 1045–1052 (2009).
Palma, E. et al. Genetic ablation of cyclophilin D rescues mitochondrial defects and prevents muscle apoptosis in collagen VI myopathic mice. Hum. Mol. Genet. 18, 2024–2031 (2009).
Grumati, P. et al. Autophagy is defective in collagen VI muscular dystrophies, and its reactivation rescues myofiber degeneration. Nat. Med. 16, 1313–1320 (2010).
Chiarugi, P. & Giannoni, E. Anoikis: a necessary death program for anchorage-dependent cells. Biochem. Pharmacol. 76, 1352–1364 (2008).
Girgenrath, M., Dominov, J. A., Kostek, C. A. & Miller, J. B. Inhibition of apoptosis improves outcome in a model of congenital muscular dystrophy. J. Clin. Invest. 114, 1635–1639 (2004).
Miller, J. B. & Girgenrath, M. The role of apoptosis in neuromuscular diseases and prospects for anti-apoptosis therapy. Trends Mol. Med. 12, 279–286 (2006).
Hayashi, Y. K. et al. Massive muscle cell degeneration in the early stage of merosin-deficient congenital muscular dystrophy. Neuromuscul. Disord. 11, 350–359 (2001).
Kawahara, G. et al. Diminished binding of mutated collagen VI to the extracellular matrix surrounding myocytes. Muscle Nerve 38, 1192–1195 (2008).
Lampe, A. K. et al. Exon skipping mutations in collagen VI are common and are predictive for severity and inheritance. Hum. Mutat. 29, 809–822 (2008).
Foley, A. R. et al. Autosomal recessive inheritance of classic Bethlem myopathy. Neuromuscul. Disord. 19, 813–817 (2009).
Gualandi, F. et al. Autosomal recessive Bethlem myopathy. Neurology 73, 1883–1891 (2009).
Camacho Vanegas, O. et al. Ullrich scleroatonic muscular dystrophy is caused by recessive mutations in collagen type VI. Proc. Natl Acad. Sci. USA 98, 7516–7521 (2001).
Lucarini, L. et al. A homozygous COL6A2 intron mutation causes in-frame triple-helical deletion and nonsense-mediated mRNA decay in a patient with Ullrich congenital muscular dystrophy. Hum. Genet. 117, 460–466 (2005).
Lampe, A. K. et al. Automated genomic sequence analysis of the three collagen VI genes: applications to Ullrich congenital muscular dystrophy and Bethlem myopathy. J. Med. Genet. 42, 108–120 (2005).
Demir, E. et al. Mutations in COL6A3 cause severe and mild phenotypes of Ullrich congenital muscular dystrophy. Am. J. Hum. Genet. 70, 1446–1458 (2002).
Giusti, B. et al. Dominant and recessive COL6A1 mutations in Ullrich scleroatonic muscular dystrophy. Ann. Neurol. 58, 400–410 (2005).
Pepe, G. et al. COL6A1 genomic deletions in Bethlem myopathy and Ullrich muscular dystrophy. Ann. Neurol. 59, 190–195 (2006).
Bovolenta, M. et al. Identification of a deep intronic mutation in the COL6A2 gene by a novel custom oligonucleotide CGH array designed to explore allelic and genetic heterogeneity in collagen VI-related myopathies. BMC Med. Genet. 11, 44 (2010).
Zhang, R. Z. et al. Recessive COL6A2 C-globular missense mutations in Ullrich congenital muscular dystrophy: role of the C2a splice variant. J. Biol. Chem. 285, 10005–10015 (2010).
Pepe, G. et al. A heterozygous splice site mutation in COL6A1 leading to an in-frame deletion of the α1(VI) collagen chain in an italian family affected by Bethlem myopathy. Biochem. Biophys. Res. Commun. 258, 802–807 (1999).
Vanegas, O. C. et al. Novel COL6A1 splicing mutation in a family affected by mild Bethlem myopathy. Muscle Nerve 25, 513–519 (2002).
Lucioli, S. et al. Detection of common and private mutations in the COL6A1 gene of patients with Bethlem myopathy. Neurology 64, 1931–1937 (2005).
Jöbsis, G. J. et al. Type VI collagen mutations in Bethlem myopathy, an autosomal dominant myopathy with contractures. Nat. Genet. 14, 113–115 (1996).
Pepe, G. et al. A novel de novo mutation in the triple helix of the COL6A3 gene in a two-generation Italian family affected by Bethlem myopathy. A diagnostic approach in the mutations' screening of type VI collagen. Neuromuscul. Disord. 9, 264–271 (1999).
Byers, P. H. Folding defects in fibrillar collagens. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356, 151–158 (2001).
Lamandé, S. R. et al. Kinked collagen VI tetramers and reduced microfibril formation as a result of Bethlem myopathy and introduced triple helical glycine mutations. J. Biol. Chem. 277, 1949–1956 (2002).
Pace, R. A. et al. Collagen VI glycine mutations: perturbed assembly and a spectrum of clinical severity. Ann. Neurol. 64, 294–303 (2008).
Petrini, S. et al. Ullrich myopathy phenotype with secondary ColVI defect identified by confocal imaging and electron microscopy analysis. Neuromuscul. Disord. 17, 587–596 (2007).
Allamand, V., Merlini, L. & Bushby, K. 166th ENMC International Workshop on Collagen type VI-related Myopathies, 22–24 May 2009, Naarden, The Netherlands. Neuromuscul. Disord. 20, 346–354 (2010).
Wang, C. H. et al. Consensus statement on standard of care for congenital muscular dystrophies. J. Child. Neurol. 25, 1559–1581 (2010).
Merlini, L. et al. Cyclosporin A corrects mitochondrial dysfunction and muscle apoptosis in patients with collagen VI myopathies. Proc. Natl Acad. Sci. USA 105, 5225–5229 (2008).
Hicks, D. et al. Cyclosporine A treatment for Ullrich congenital muscular dystrophy: a cellular study of mitochondrial dysfunction and its rescue. Brain 132, 147–155 (2009).
Millay, D. P. et al. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat. Med. 14, 442–447 (2008).
Collins, J. & Bönnemann, C. G. Congenital muscular dystrophies: toward molecular therapeutic interventions. Curr. Neurol. Neurosci. Rep. 10, 83–91 (2010).
Bethlem, J. & Wijngaarden, G. K. Benign myopathy, with autosomal dominant inheritance. A report on three pedigrees. Brain 99, 91–100 (1976).
Mohire, M. D. et al. Early-onset benign autosomal dominant limb-girdle myopathy with contractures (Bethlem myopathy). Neurology 38, 573–580 (1988).
Jöbsis, G. J. et al. Genetic localization of Bethlem myopathy. Neurology 46, 779–782 (1996).
Pan, T. C. et al. Missense mutation in a von Willebrand factor type A domain of the α3(VI) collagen gene (COL6A3) in a family with Bethlem myopathy. Hum. Mol. Genet. 7, 807–812 (1998).
Bidanset, D. J. et al. Binding of the proteoglycan decorin to collagen type VI. J. Biol. Chem. 267, 5250–5256 (1992).
Sasaki, T., Gohring, W., Pan, T. C., Chu, M. L. & Timpl, R. Binding of mouse and human fibulin-2 to extracellular matrix ligands. J. Mol. Biol. 254, 892–899 (1995).
Tillet, E. et al. Recombinant expression and structural and binding properties of α1(VI) and α2(VI) chains of human collagen type VI. Eur. J. Biochem. 221, 177–185 (1994).
Finnis, M. L. & Gibson, M. A. Microfibril-associated glycoprotein-1 (MAGP-1) binds to the pepsin-resistant domain of the α3(VI) chain of type VI collagen. J. Biol. Chem. 272, 22817–22823 (1997).
Specks, U. et al. Structure of recombinant N-terminal globule of type VI collagen alpha 3 chain and its binding to heparin and hyaluronan. EMBO J. 11, 4281–4290 (1992).
Brown, J. C., Mann, K., Wiedemann, H. & Timpl, R. Structure and binding properties of collagen type XIV isolated from human placenta. J. Cell Biol. 120, 557–567 (1993).
Burg, M. A., Tillet, E., Timpl, R. & Stallcup, W. B. Binding of the NG2 proteoglycan to type VI collagen and other extracellular matrix molecules. J. Biol. Chem. 271, 26110–26116 (1996).
Stallcup, W. B., Dahlin, K. & Healy, P. Interaction of the NG2 chondroitin sulfate proteoglycan with type VI collagen. J. Cell Biol. 111, 3177–3188 (1990).
Nishiyama, A. & Stallcup, W. B. Expression of NG2 proteoglycan causes retention of type VI collagen on the cell surface. Mol. Biol. Cell 4, 1097–1108 (1993).
Wiberg, C., Heinegard, D., Wenglen, C., Timpl, R. & Morgelin, M. Biglycan organizes collagen VI into hexagonal-like networks resembling tissue structures. J. Biol. Chem. 277, 49120–49126 (2002).
Wiberg, C. et al. Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan. J. Biol. Chem. 278, 37698–37704 (2003).
Pfaff, M. et al. Integrin and Arg-Gly-Asp dependence of cell adhesion to the native and unfolded triple helix of collagen type VI. Exp. Cell Res. 206, 167–176 (1993).
Acknowledgements
The author would like to thank members of his laboratory and participants of the 166th ENMC International Workshop on Collagen type VI-related Myopathies, for helpful discussions.
L. Barclay, freelance writer and reviewer, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape, LLC-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Bönnemann, C. The collagen VI-related myopathies: muscle meets its matrix. Nat Rev Neurol 7, 379–390 (2011). https://doi.org/10.1038/nrneurol.2011.81
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2011.81
This article is cited by
-
Dry synovitis, a rare entity distinct from juvenile idiopathic arthritis
Pediatric Rheumatology (2023)
-
Contextual fear memory impairment in Angelman syndrome model mice is associated with altered transcriptional responses
Scientific Reports (2023)
-
Decellularized Extracellular Matrix-Derived Hydrogels: a Powerful Class of Biomaterials for Skeletal Muscle Regenerative Engineering Applications
Regenerative Engineering and Translational Medicine (2023)
-
Study of the collagen type VI alpha 3 (COL6A3) gene in Parkinson’s disease
BMC Neurology (2021)
-
Causative variant profile of collagen VI-related dystrophy in Japan
Orphanet Journal of Rare Diseases (2021)