Abstract
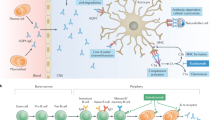
Antibodies to aquaporin-4 (also known as AQP4-Ab or NMO-IgG) are sensitive and highly specific serum markers of autoimmune neuromyelitis optica (NMO). Second-generation recombinant diagnostic assays can detect AQP4-Ab in ≥80% of patients with NMO, and a role for AQP4-Ab in the pathophysiology of this condition was corroborated by a series of in vitro studies that demonstrated disruption of the blood–brain barrier, impairment of glutamate homeostasis and induction of necrotic cell death by AQP4-Ab-positive serum. Additional evidence for such a role has emerged from clinical observations, including the demonstration of a correlation between serum levels of AQP4-Ab and disease activity. The finding of NMO-like CNS lesions and clinical disease following passive transfer of AQP4-Ab-positive serum in several independent animal studies provided definitive proof for a pathogenic role of AQP4-Ab in vivo. Together, these findings provide a strong rationale for the use of therapies targeted against B cells or antibodies in the treatment of NMO. In this Review, we summarize the latest evidence in support of a direct involvement of AQP4-Ab in the immunopathogenesis of NMO, and critically appraise the diagnostic tests currently available for the detection of this serum reactivity.
Key Points
-
Growing evidence implicates aquaporin-4 antibody (AQP4-Ab)-mediated autoimmunity in the pathogenesis of neuromyelitis optica (NMO) and its formes frustes, but not in multiple sclerosis or other CNS autoimmune conditions
-
Robust evidence for a direct involvement of AQP4-Ab in NMO immunopathogenesis comes from animal studies reporting NMO-like disease after passive transfer of AQP4-Ab-positive human serum
-
A strong rationale exists for the use of therapies targeting B cells or antibodies in NMO, and this will be an important consideration for future treatment trials
-
Second-generation recombinant serological assays with higher sensitivity and specificity than the original 'NMO-IgG' test are now available and should be offered to all patients with NMO or related disorders
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wingerchuk, D. M., Hogancamp, W. F., O'Brien, P. C. & Weinshenker, B. G. The clinical course of neuromyelitis optica (Devic's syndrome). Neurology 53, 1107–1114 (1999).
de Seze, J. et al. Devic's neuromyelitis optica: clinical, laboratory, MRI and outcome profile. J. Neurol. Sci. 197, 57–61 (2002).
Jarius, S. et al. Mechanisms of disease: aquaporin-4 antibodies in neuromyelitis optica. Nat. Clin. Pract. Neurol. 4, 202–214 (2008).
Lucchinetti, C. F. et al. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain 125, 1450–1461 (2002).
Weinshenker, B. G. et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann. Neurol. 46, 878–886 (1999).
Keegan, M. et al. Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology 58, 143–146 (2002).
Lennon, V. A. et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 364, 2106–2112 (2004).
Lennon, V. A., Kryzer, T. J., Pittock, S. J., Verkman, A. S. & Hinson, S. R. IgG marker of optic–spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 202, 473–477 (2005).
Roemer, S. F. et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain 130, 1194–1205 (2007).
Misu, T. et al. Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain 130, 1224–1234 (2007).
Weinshenker, B. G. et al. Neuromyelitis optica IgG predicts relapse after longitudinally extensive transverse myelitis. Ann. Neurol. 59, 566–569 (2006).
Matiello, M. et al. NMO-IgG predicts the outcome of recurrent optic neuritis. Neurology 70, 2197–2200 (2008).
Takahashi, T. et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain 130, 1235–1243 (2007).
Jarius, S. et al. Antibody to aquaporin-4 in the long-term course of neuromyelitis optica. Brain 131, 3072–3080 (2008).
Bonnan, M. et al. Plasma exchange in severe spinal attacks associated with neuromyelitis optica spectrum disorder. Mult. Scler. 15, 487–492 (2009).
Watanabe, S. et al. Therapeutic efficacy of plasma exchange in NMO-IgG-positive patients with neuromyelitis optica. Mult. Scler. 13, 128–132 (2007).
Nakashima, I. et al. Plasma exchange for neuromyelitis optica with aquaporin-4 antibody [abstract P04.092]. Neurology 72 (Suppl. 3), A187 (2009).
Cree, B. A. et al. An open label study of the effects of rituximab in neuromyelitis optica. Neurology 64, 1270–1272 (2005).
Jacob, A. et al. Treatment of neuromyelitis optica with rituximab: retrospective analysis of 25 patients. Arch. Neurol. 65, 1443–1448 (2008).
Bedi, G. S., Brown, A., Delgado, S., Usmani, N. & Sheremata, W. Effect of rituximab on relapse rate and disability in neuromyelitis optica (NMO) [abstract P04.099]. Neurology 72 (Suppl. 3), A189 (2009).
Heerlein, K. et al. Aquaporin-4 antibody positive longitudinally extensive transverse myelitis following varicella zoster infection. J. Neurol. Sci. 276, 184–186 (2009).
Suzuki, C. et al. Anti-aquaporin-4 antibody production from peripheral B cells in neuromyelitis optica [abstract S39.005]. Neurology 72 (Suppl. 3), A281 (2009).
Meinl, E., Krumbholz, M. & Hohlfeld, R. B lineage cells in the inflammatory central nervous system environment: migration, maintenance, local antibody production, and therapeutic modulation. Ann. Neurol. 59, 880–892 (2006).
Sinclair, C., Kirk, J., Herron, B., Fitzgerald, U. & McQuaid, S. Absence of aquaporin-4 expression in lesions of neuromyelitis optica but increased expression in multiple sclerosis lesions and normal-appearing white matter. Acta Neuropathol. (Berl.) 113, 187–194 (2007).
Misu, T. et al. Loss of aquaporin-4 in active perivascular lesions in neuromyelitis optica: a case report. Tohoku J. Exp. Med. 209, 269–275 (2006).
Saadoun, S. et al. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain 133, 349–361 (2010).
Yanagawa, K. et al. Pathologic and immunologic profiles of a limited form of neuromyelitis optica with myelitis. Neurology 73, 1628–1637 (2009).
Kale, N. et al. Humoral pattern II multiple sclerosis pathology not associated with neuromyelitis optica IgG. Arch. Neurol. 66, 1298–1299 (2009).
Misu, T. et al. Marked increase in cerebrospinal fluid glial fibrillar acidic protein in neuromyelitis optica: an astrocytic damage marker. J. Neurol. Neurosurg. Psychiatry 80, 575–577 (2009).
Miyazawa, I. et al. High CSF neurofilament heavy chain levels in neuromyelitis optica. Neurology 68, 865–867 (2007).
Uzawa, A. et al. Markedly increased CSF interleukin-6 levels in neuromyelitis optica, but not in multiple sclerosis. J. Neurol. 256, 2082–2084 (2009).
Okada, K., Matsushita, T., Kira, J. & Tsuji, S. B-cell activating factor of the TNF family is upregulated in neuromyelitis optica. Neurology 74, 177–178 (2010).
Icoz, S. et al. Enhanced IL-6 production in aquaporin-4 antibody positive neuromyelitis optica patients. Int. J. Neurosci. 120, 71–75 (2010).
Alves-Leon, S. V. et al. Immune system markers of neuroinflammation in patients with clinical diagnose of neuromyelitis optica. Arq. Neuropsiquiatr. 66, 678–684 (2008).
Matsushita, T. et al. Aquaporin-4 autoimmune syndrome and anti-aquaporin-4 antibody-negative opticospinal multiple sclerosis in Japanese. Mult. Scler. 15, 834–847 (2009).
Correale, J. & Fiol, M. Activation of humoral immunity and eosinophils in neuromyelitis optica. Neurology 63, 2363–2370 (2004).
Hinson, S. R. et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology 69, 2221–2231 (2007).
Vincent, T. et al. Functional consequences of neuromyelitis optica-IgG astrocyte interactions on blood–brain barrier permeability and granulocyte recruitment. J. Immunol. 181, 5730–5737 (2008).
Hinson, S. R. et al. Aquaporin-4-binding autoantibodies in patients with neuromyelitis optica impair glutamate transport by down-regulating EAAT2. J. Exp. Med. 205, 2473–2481 (2008).
Kinoshita, M. et al. Anti-aquaporin-4 antibody induces astrocytic cytotoxicity in the absence of CNS antigen-specific T cells. Biochem. Biophys. Res. Commun. 394, 205–210 (2010).
Zhou, J. et al. Altered blood–brain barrier integrity in adult aquaporin-4 knockout mice. Neuroreport 19, 1–5 (2008).
Matsushita, T. et al. Extensive vasogenic edema of anti-aquaporin-4 antibody-related brain lesions. Mult. Scler. 15, 1113–1117 (2009).
Zeng, X. N. et al. Aquaporin-4 deficiency down-regulates glutamate uptake and GLT-1 expression in astrocytes. Mol. Cell. Neurosci. 34, 34–39 (2007).
Matute, C. et al. The link between excitotoxic oligodendroglial death and demyelinating diseases. Trends Neurosci. 24, 224–230 (2001).
Alberdi, E. et al. Activation of kainate receptors sensitizes oligodendrocytes to complement attack. J. Neurosci. 26, 3220–3228 (2006).
Waters, P. et al. Aquaporin-4 antibodies in neuromyelitis optica and longitudinally extensive transverse myelitis. Arch. Neurol. 65, 913–919 (2008).
Kalluri, S. R. et al. A novel bio-assay to determine and characterize the antibodies to aquaporin-4 [abstract P04.102]. Neurology 72 (Suppl. 3), A189 (2009).
Matsushita, T. et al. Aquaporin-4 autoimmune syndrome and anti-aquaporin-4 antibody-negative opticospinal multiple sclerosis in Japanese. Mult. Scler. 15, 834–847 (2009).
Spiller, O. B., Moretto, G., Kim, S. U., Morgan, B. P. & Devine, D. V. Complement expression on astrocytes and astrocytoma cell lines: failure of complement regulation at the C3 level correlates with very low CD55 expression. J. Neuroimmunol. 71, 97–106 (1996).
Kinoshita, M. et al. Astrocytic necrosis is induced by anti-aquaporin-4 antibody-positive serum. Neuroreport 20, 508–512 (2009).
Sabater, L. et al. Cytotoxic effect of neuromyelitis optica antibody (NMO-IgG) to astrocytes: an in vitro study. J. Neuroimmunol. 215, 31–35 (2009).
Hinson, S. R. et al. Prediction of neuromyelitis optica attack severity by quantitation of complement-mediated injury to aquaporin-4-expressing cells. Arch. Neurol. 66, 1164–1167 (2009).
Doi, H. et al. Hypercomplementemia at relapse in patients with anti-aquaporin-4 antibody. Mult. Scler. 15, 304–310 (2009).
Jarius, S., Franciotta, D., Bergamaschi, R., Wildemann, B. & Wandinger, K. P. Immunoglobulin M antibodies to aquaporin-4 in neuromyelitis optica and related disorders. Clin. Chem. Lab. Med. doi: 10.1515/CCLM.2010.127.
Saikali, P., Cayrol, R. & Vincent, T. Anti-aquaporin-4 auto-antibodies orchestrate the pathogenesis in neuromyelitis optica. Autoimmun. Rev. 9, 132–135 (2009).
Tani, T. et al. Identification of binding sites for anti-aquaporin 4 antibodies in patients with neuromyelitis optica. J. Neuroimmunol. 211, 110–113 (2009).
Nicchia, G. P. et al. Aquaporin-4 orthogonal arrays of particles are the target for neuromyelitis optica autoantibodies. Glia 57, 1363–1373 (2009).
Furman, C. S. et al. Aquaporin-4 square array assembly: opposing actions of M1 and M23 isoforms. Proc. Natl Acad. Sci. USA 100, 13609–13614 (2003).
Matiello, M. et al. Two different Arg19 mutations in the N-terminus of aquaporin-4 suggest a molecular mechanism for susceptibility to neuromyelitis optica [abstract S17.001]. Neurology 72 (Suppl. 3), A119 (2009).
Crane, J. M., Bennett, J. L. & Verkman, A. S. Live cell analysis of aquaporin-4 M1/M23 interactions and regulated orthogonal array assembly in glial cells. J. Biol. Chem. 284, 35850–35860 (2009).
Jarius, S. et al. Standardized method for the detection of antibodies to aquaporin-4 based on a highly sensitive immunofluorescence assay employing recombinant target antigen. J. Neurol. Sci. 291, 52–56 (2010).
Paul, F. et al. Antibody to aquaporin 4 in the diagnosis of neuromyelitis optica. PLoS Med. 4, e133 (2007).
Marnetto, F. et al. Western blot analysis for the detection of serum antibodies recognizing linear aquaporin-4 epitopes in patients with neuromyelitis optica. J. Neuroimmunol. 217, 74–79 (2009).
Kinoshita, M. et al. Neuromyelitis optica: passive transfer to rats by human immunoglobulin. Biochem. Biophys. Res. Commun. 386, 623–627 (2009).
Bradl, M. et al. Neuromyelitis optica: Pathogenicity of patient immunoglobulin in vivo. Ann. Neurol. 66, 630–643 (2009).
Bennett, J. L. et al. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann. Neurol. 66, 617–629 (2009).
Nishiyama, S. et al. A case of NMO seropositive for aquaporin-4 antibody more than 10 years before onset. Neurology 72, 1960–1961 (2009).
Jarius, S. et al. Neuromyelitis optica and longitudinally extensive transverse myelitis following thymectomy for myasthenia gravis [abstract P534]. Mult. Scler. 13 (Suppl. 2), S159 (2007).
Ahasan, H. A., Rafiqueuddin, A. K., Chowdhury, M. A., Azhar, M. A. & Kabir, F. Neuromyelitis optica (Devic's disease) following chicken pox. Trop. Doct. 24, 75–76 (1994).
Chusid, M. J., Williamson, S. J., Murphy, J. V. & Ramey, L. S. Neuromyelitis optica (Devic disease) following varicella infection. J. Pediatr. 95, 737–738 (1979).
Tran, C. et al. Neuromyelitis optica following CMV primo-infection. J. Intern. Med. 261, 500–503 (2007).
Ghezzi, A. et al. Clinical characteristics, course and prognosis of relapsing Devic's neuromyelitis optica. J. Neurol. 251, 47–52 (2004).
Jarius, S. et al. NMO-IgG in the diagnosis of neuromyelitis optica. Neurology 68, 1076–1077 (2007).
Weinshenker, B. G., Wingerchuk, D. M., Nakashima, I., Fujihara, K. & Lennon, V. A. OSMS is NMO, but not MS: proven clinically and pathologically. Lancet Neurol. 5, 110–111 (2006).
Matsuoka, T. et al. Heterogeneity of aquaporin-4 autoimmunity and spinal cord lesions in multiple sclerosis in Japanese. Brain 130, 1206–1223 (2007).
Takahashi, T. et al. Establishment of a new sensitive assay for anti-human aquaporin-4 antibody in neuromyelitis optica. Tohoku J. Exp. Med. 210, 307–313 (2006).
Nakashima, I. et al. Clinical and MRI features of Japanese patients with multiple sclerosis positive for NMO-IgG. J. Neurol. Neurosurg. Psychiatry 77, 1073–1075 (2006).
Petzold, A. et al. Neuromyelitis optica-IgG (aquaporin-4) autoantibodies in immune mediated optic neuritis. J. Neurol. Neurosurg. Psychiatry 81, 109–111 (2010).
Takahashi, T. et al. Intractable hiccup and nausea in neuromyelitis optica with anti-aquaporin-4 antibody: a herald of acute exacerbations. J. Neurol. Neurosurg. Psychiatry 79, 1075–1078 (2008).
Pittock, S. J. et al. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch. Neurol. 63, 964–968 (2006).
Magana, S. M. et al. Posterior reversible encephalopathy syndrome in neuromyelitis optica spectrum disorders. Neurology 72, 712–717 (2009).
Ito, S. Posterior reversible encephalopathy syndrome in neuromyelitis optica spectrum disorders. Neurology 73, 1604 (2009).
McKeon, A. et al. CNS aquaporin-4 autoimmunity in children. Neurology 71, 93–100 (2008).
Wandinger, K. P. et al. Autoantibodies against aquaporin-4 in patients with neuropsychiatric SLE and primary Sjögren´s syndrome. Arthritis Rheum. 62, 1198–1200 (2010).
Pittock, S. J. et al. Neuromyelitis optica and non organ-specific autoimmunity. Arch. Neurol. 65, 78–83 (2008).
Hayakawa, S. et al. Neuromyelitis optica and anti-aquaporin-4 antibodies measured by an enzyme-linked immunosorbent assay. J. Neuroimmunol. 196, 181–187 (2008).
Pittock, S. J. & Lennon, V. A. Aquaporin-4 autoantibodies in a paraneoplastic context. Arch. Neurol. 65, 629–632 (2008).
Cross, S. A. et al. Paraneoplastic autoimmune optic neuritis with retinitis defined by CRMP-5-IgG. Ann. Neurol. 54, 38–50 (2003).
Ducray, F. et al. Devic's syndrome-like phenotype associated with thymoma and anti-CV2/CRMP5 antibodies. J. Neurol. Neurosurg. Psychiatry 78, 325–327 (2007).
Klawiter, E. C. et al. NMO-IgG detected in CSF in seronegative neuromyelitis optica. Neurology 72, 1101–1103 (2009).
Cree, B. A., Goodin, D. S. & Hauser, S. L. Neuromyelitis optica. Semin. Neurol. 22, 105–122 (2002).
Ho, J. D. et al. Crystal structure of human aquaporin 4 at 1.8 Å and its mechanism of conductance. Proc. Natl Acad. Sci. USA 106, 7437–7442 (2009).
Moreland, J. L., Gramada, A., Buzko, O. V., Zhang, Q. & Bourne, P. E. The Molecular Biology Toolkit (MBT): a modular platform for developing molecular visualization applications. BMC Bioinformatics 6, 21 (2005).
Adoni, T., Lino, A. M., Marchiori, P. E., Kok, F. & Callegaro, D. Seroprevalence of NMO-IgG antibody in Brazilian patients with neuromyelitis optica. Arq. Neuropsiquiatr. 66, 295–297 (2008).
Marignier, R. et al. NMO-IgG and Devic's neuromyelitis optica: a French experience. Mult. Scler. 14, 440–445 (2008).
Saiz, A. et al. Revised diagnostic criteria for neuromyelitis optica (NMO): application in a series of suspected patients. J. Neurol. 254, 1233–1237 (2007).
Bizzoco, E. et al. Prevalence of neuromyelitis optica spectrum disorder and phenotype distribution. J. Neurol. 256, 1891–1898 (2009).
Wingerchuk, D. M., Lennon, V. A., Pittock, S. J., Lucchinetti, C. F. & Weinshenker, B. G. Revised diagnostic criteria for neuromyelitis optica. Neurology 66, 1485–1489 (2006).
Apiwattanakul, M. et al. AQP4-IgG immunoprecipitation assay optimization. Clin. Chem. 55, 592–594 (2009).
Acknowledgements
The work of S. Jarius was supported by a Research Fellowship from the European Committee for Research and Treatment in Multiple Sclerosis (ECTRIMS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have received research grants from Bayer Schering Pharma and Merck Serono. The funding sources had no role in data collection, analysis of data, preparation of the manuscript, or decision to publish this Review.
Rights and permissions
About this article
Cite this article
Jarius, S., Wildemann, B. AQP4 antibodies in neuromyelitis optica: diagnostic and pathogenetic relevance. Nat Rev Neurol 6, 383–392 (2010). https://doi.org/10.1038/nrneurol.2010.72
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2010.72
This article is cited by
-
Methyl-CpG-Binding Protein 2 Emerges as a Central Player in Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorders
Cellular and Molecular Neurobiology (2023)
-
Update on the diagnosis and treatment of neuromyelits optica spectrum disorders (NMOSD) – revised recommendations of the Neuromyelitis Optica Study Group (NEMOS). Part I: Diagnosis and differential diagnosis
Journal of Neurology (2023)
-
Inositol 1,4,5-trisphosphate receptor type 1 autoantibody (ITPR1-IgG/anti-Sj)-associated autoimmune cerebellar ataxia, encephalitis and peripheral neuropathy: review of the literature
Journal of Neuroinflammation (2022)
-
Comparative analysis of clinical and imaging data between patients with myelin oligodendrocyte glycoprotein antibody disease and patients with aquaporin 4 antibody-positive neuromyelitis optica spectrum disorder
Journal of Neurology (2022)
-
P2R Inhibitors Prevent Antibody-Mediated Complement Activation in an Animal Model of Neuromyelitis Optica
Neurotherapeutics (2022)