Abstract
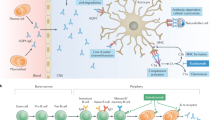
Neuromyelitis optica (NMO) is a rare CNS inflammatory disorder that predominantly affects the optic nerves and spinal cord. Recent serological findings strongly suggest that NMO is a distinct disease rather than a subtype of multiple sclerosis. In NMO, serum antibodies, collectively known as NMO-IgG, characteristically bind to cerebral microvessels, pia mater and Virchow–Robin spaces. The main target antigen for this immunoreactivity has been identified as aquaporin-4 (AQP4). The antibodies are highly specific for NMO, and they are also found in patients with longitudinally extensive transverse myelitis without optic neuritis, which is thought to be a precursor to NMO in some cases. An antibody-mediated pathogenesis for NMO is supported by several observations, including the characteristics of the AQP4 antibodies, the distinct NMO pathology—which includes IgG and complement deposition and loss of AQP4 from spinal cord lesions—and emerging evidence of the beneficial effects of B-cell depletion and plasma exchange. Many aspects of the pathogenesis, however, remain unclear.
Key Points
-
Neuromyelitis optica (NMO) is an inflammatory disorder of the CNS of putative autoimmune etiology that predominantly affects the spinal cord and optic nerves
-
NMO is histologically characterized by extensive demyelination and substantial axonal damage; the presence of IgG and complement deposits suggests a humoral pathogenesis
-
Recently, a new serum reactivity (called NMO-IgG), characterized by binding of IgG to structures adjacent to the microvasculature and pia mater, has been detected in patients with NMO
-
Aquaporin-4, the most abundant water channel in the CNS, has been identified as the target antigen of NMO-IgG
-
Indirect evidence from immunobiological and histological studies suggests an important role for NMO-IgG/AQP4-Ab in the pathogenesis of NMO
-
These new findings facilitate the diagnosis of NMO and might soon translate into new therapeutic approaches
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Devic E (1894) Subacute myelitis complicated by optic neuritis [French]. Bull Med 8: 1033–1034
Devic E (1895) Acute dorsolumbar myelitis with optic neuritis, autopsy [French]. Congress Francais Medicine 1: 434–439
Allbutt TC (1870) On the ophthalmoscopic signs of spinal disease. Lancet 1: 76–88
Erb W (1879) About the concurrence of optic neuritis and subacute myelitis [German]. Arch Psychiatr Nervenkr 1: 146–157
Lucchinetti CF et al. (2002) A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain 125: 1450–1461
Lennon VA et al. (2004) A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 364: 2106–2112
Lennon VA et al. (2005) IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 202: 473–477
Paul F et al. (2007) Antibody to aquaporin-4 in the diagnosis of neuromyelitis optica. PLoS Med 4: e133
Jarius S et al. (2007) NMO-IgG in the diagnosis of neuromyelitis optica. Neurology 68: 1076–1077
Wingerchuk DM et al. (2006) Revised diagnostic criteria for neuromyelitis optica. Neurology 66: 1485–1489
Wingerchuk DM et al. (1999) The clinical course of neuromyelitis optica (Devic's syndrome). Neurology 53: 1107–1114
Pittock SJ et al. (2006) Brain abnormalities in neuromyelitis optica. Arch Neurol 63: 390–396
de Seze J et al. (2002) Devic's neuromyelitis optica: clinical, laboratory, MRI and outcome profile. J Neurol Sci 197: 57–61
Wingerchuk DM et al. (2007) A secondary progressive clinical course is uncommon in neuromyelitis optica. Neurology 68: 603–605
Ghezzi A et al. (2004) Clinical characteristics, course and prognosis of relapsing Devic's neuromyelitis optica. J Neurol 251: 47–52
O'Riordan JI et al. (1996) Clinical, CSF, and MRI findings in Devic's neuromyelitis optica. J Neurol Neurosurg Psychiatry 60: 382–387
Nakashima I et al. (2006) Clinical and MRI features of Japanese patients with multiple sclerosis positive for NMO-IgG. J Neurol Neurosurg Psychiatry 77: 1073–1075
Jeffery AR and Buncic JR (1996) Pediatric Devic's neuromyelitis optica. J Pediatr Ophthalmol Strabismus 33: 223–229
Filley CM et al. (1984) Neuromyelitis optica in the elderly. Arch Neurol 41: 670–672
Filippi M and Rocca MA (2004) MR imaging of Devic's neuromyelitis optica. Neurol Sci 25 (Suppl 4): S371–S373
Lycklama G et al. (2000) Spinal-cord MRI in multiple sclerosis. Lancet Neurol 2: 555–562
Tartaglino LM et al. (1995) Multiple sclerosis in the spinal cord: MR appearance and correlation with clinical parameters. Radiology 195: 725–732
Lin F et al. (2006) Discriminative analysis of relapsing neuromyelitis optica and relapsing–remitting multiple sclerosis based on two-dimensional histogram from diffusion tensor imaging. Neuroimage 31: 543–549
Benedetti B et al. (2006) Grading cervical cord damage in neuromyelitis optica and MS by diffusion tensor MRI. Neurology 67: 161–163
Green AJ et al. (2007) Funduscopic and optical coherence tomography findings in neuromyelitis optica compared to multiple sclerosis. Neurology 68 (Suppl 1): SA355
Cabrera-Gomez JA et al. (2007) Brain magnetic resonance imaging findings in relapsing neuromyelitis optica. Mult Scler 13: 186–192
Pittock SJ et al. (2006) Neuromyelitis optica brain lesions localized at sites of high aquaporin-4 expression. Arch Neurol 63: 964–968
Zaffaroni M (2004) Cerebrospinal fluid findings in Devic's neuromyelitis optica. Neurol Sci 25 (Suppl 4): S368–S370
Bergamaschi R et al. (2004) Oligoclonal bands in Devic's neuromyelitis optica and multiple sclerosis: differences in repeated cerebrospinal fluid examinations. Mult Scler 10: 2–4
Melamud L et al. (2006) Cerebrospinal fluid findings in Devic's neuromyelitis optica. Mult Scler 12: P246
Reiber H et al. (1998) The intrathecal, polyspecific and oligoclonal immune response in multiple sclerosis. Mult Scler 4: 111–117
Meinl E et al. (2006) B lineage cells in the inflammatory central nervous system environment: migration, maintenance, local antibody production, and therapeutic modulation. Ann Neurol 59: 880–892
Jarius S et al. Polyspecific, antiviral immune response distinguishes multiple sclerosis and neuromyelitis optica. J Neurol Neurosurg Psychiatry, in press
Satoh J et al. (2003) Detection of the 14-3-3 protein in the cerebrospinal fluid of Japanese multiple sclerosis patients presenting with severe myelitis. J Neurol Sci 212: 11–20
Petzold A (2007) NMO-IgG serology in chronic relapsing inflammatory optic neuropathy. Mult Scler 13 (Suppl 2): SP538
Matiello M (2007) NMO-IgG predicts the outcome of recurrent optic neuritis. Mult Scler 13 (Suppl 2): SP536
Jacobi C et al. (2006) Neuromyelitis optica (Devic's syndrome) as first manifestation of systemic lupus erythematosus. Lupus 15: 107–109
Weinshenker B et al. (2006) The relationship between neuromyelitis optica and systemic auto-immune disease. Mult Scler 12: O79
Scott TF et al. (2006) Neuromyelitis optica IgG status in acute partial transverse myelitis. Arch Neurol 63: 1398–1400
Takahashi T et al. (2007) Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain 130: 1235–1243
Yang B et al. (1995) cDNA cloning, gene organization, and chromosomal localization of a human mercurial insensitive water channel: evidence for distinct transcriptional units. J Biol Chem 270: 22907–22913
Lu M et al. (1996) The human AQP4 gene: definition of the locus encoding two water channel polypeptides in brain. Proc Natl Acad Sci USA 93: 10908–10912
Jung JS et al. (1994) Molecular characterization of an aquaporin cDNA from brain: candidate osmoreceptor and regulator of water balance. Proc Natl Acad Sci USA 91: 13052–13056
Hiroaki Y et al. (2006) Implications of the aquaporin-4 structure on array formation and cell adhesion. J Mol Biol 355: 628–639
Amiry-Moghaddam M and Ottersen OP (2003) The molecular basis of water transport in the brain. Nat Rev Neurosci 4: 991–1001
Nielsen S et al. (1997) Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci 17: 171–180
Roemer SF et al. (2007) Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain 130: 1194–1205
Watanabe S et al. (2007) Low-dose corticosteroids reduce relapses in neuromyelitis optica: a retrospective analysis. Mult Scler 13: 968–974
Ma T et al. (1997) Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. J Clin Invest 100: 957–962
Amiry-Moghaddam M et al. (2004) Anchoring of aquaporin-4 in brain: molecular mechanisms and implications for the physiology and pathophysiology of water transport. Neuroscience 129: 999–1010
Hoch W (1999) Formation of the neuromuscular junction: agrin and its unusual receptors. Eur J Biochem 265: 1–10
Manley GT et al. (2000) Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med 6: 159–163
Amiry-Moghaddam M et al. (2003) Delayed K+ clearance associated with aquaporin-4 mislocalization: phenotypic defects in brains of α-syntrophin-null mice. Proc Natl Acad Sci USA 100: 13615–13620
Vincent A (2002) Unravelling the pathogenesis of myasthenia gravis. Nat Rev Immunol 2: 797–804
Compston A (2004) 'The marvellous harmony of the nervous parts': the origins of multiple sclerosis. Clin Med 4: 346–354
Weinshenker BG et al. (2006) OSMS is NMO, but not MS: proven clinically and pathologically. Lancet Neurol 5: 110–111
Ito H et al. (1998) HLA-DP-associated susceptibility to the optico-spinal form of multiple sclerosis in the Japanese. Tissue Antigens 52: 179–182
Kira J et al. (1996) Western versus Asian types of multiple sclerosis: immunogenetically and clinically distinct disorders. Ann Neurol 40: 569–574
Fukazawa T et al. (2006) HLA-dPB1*0501 is not uniquely associated with opticospinal multiple sclerosis in Japanese patients: important role of DPB1*0301. Mult Scler 12: 19–23
Jacob A (2007) HLA DPB1*0501 allele is not associated with neuromyelitis optica. Mult Scler 13 (Suppl 2): SP674
Cloys DE and Netsky MG (1970) Neuromyelitis optica. In Handbook of Clinical Neurology, vol 9, 426–436 (Eds Viken PJ and Bruyn GW) Amsterdam: North-Holland
Prineas JW and McDonald WI (1997) Demyelinating diseases. In Greenfield's Neuropathology, edn 6, 813–896 (Eds Graham DI and Lantos PL) London: Edward Arnold
Lucchinetti C et al. (2000) Heterogeneity of multiple sclerosis legions: implications for the pathogenesis of demyelination. Ann Neurol 47: 707–717
Mandler RN et al. (1993) Devic's neuromyelitis optica: a clinicopathological study of 8 patients. Ann Neurol 34: 162–168
Goehler LE et al. (2006) Neural–immune interface in the rat area postrema. Neuroscience 140: 1415–1434
Schulz M and Engelhardt B (2005) The circumventricular organs participate in the immunopathogenesis of experimental autoimmune encephalomyelitis. Cerebrospinal Fluid Res 2: 8
Simard M and Nedergaard M (2004) The neurobiology of glia in the context of water and ion homeostasis. Neuroscience 129: 877–896
Correale J and Fiol M (2004) Activation of humoral immunity and eosinophils in neuromyelitis optica. Neurology 63: 2363–2370
Ishizu T et al. (2005) Intrathecal activation of the IL-17/IL-8 axis in opticospinal multiple sclerosis. Brain 128: 988–1002
Takahashi T et al. (2006) Establishment of a new sensitive assay for anti-human aquaporin-4 antibody in neuromyelitis optica. Tohoku J Exp Med 210: 307–313
Hinson SR et al. (2007) Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology 69: 2221–2231
Misu T et al. (2006) Loss of aquaporin-4 in active perivascular lesions in neuromyelitis optica: a case report. Tohoku J Exp Med 209: 269–275
Aoki-Yoshino K et al. (2005) Enhanced expression of aquaporin-4 in human brain with inflammatory diseases. Acta Neuropathol 110: 281–288
Holley JE et al. (2003) Astrocyte characterization in the multiple sclerosis glial scar. Neuropathol Appl Neurobiol 29: 434–444
Ayers MM et al. (2004) Early glial responses in murine models of multiple sclerosis. Neurochem Int 45: 409–419
Auguste KI et al. (2007) Greatly impaired migration of implanted aquaporin-4-deficient astroglial cells in mouse brain toward a site of injury. FASEB J 21: 108–116
Bergamaschi R (2007) Immune agents for the treatment of Devic's neuromyelitis optica. Neurol Sci 28: 238–240
Wingerchuk DM (2007) Diagnosis and treatment of neuromyelitis optica. Neurologist 13: 2–11
Aguilera AJ et al. (1985) Lymphocytaplasmapheresis in Devic's syndrome. Transfusion 25: 54–56
Mandler RN et al. (1998) Devic's neuromyelitis optica: a prospective study of seven patients treated with prednisone and azathioprine. Neurology 51: 1219–1220
Nozaki I et al. (2006) Fulminant Devic disease successfully treated by lymphocytapheresis. J Neurol Neurosurg Psychiatry 77: 1094–1095
Weinshenker BG et al. (1999) A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol 46: 878–886
Keegan M et al. (2002) Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology 58: 143–146
Cree BA et al. (2005) An open label study of the effects of rituximab in neuromyelitis optica. Neurology 64: 1270–1272
Fidler JM et al. (1986) Selective immunomodulation by the antineoplastic agent mitoxantrone. I: suppression of B lymphocyte function. J Immunol 137: 727–732
Fidler JM et al. (1986) Selective immunomodulation by the antineoplastic agent mitoxantrone. II: nonspecific adherent suppressor cells derived from mitoxantrone-treated mice. J Immunol 136: 2747–2754
Weinstock-Guttman B et al. (2006) Study of mitoxantrone for the treatment of recurrent neuromyelitis optica (Devic disease). Arch Neurol 63: 957–963
Sinclair C et al. (2007) Absence of aquaporin-4 expression in lesions of neuromyelitis optica but increased expression in multiple sclerosis lesions and normal-appearing white matter. Acta Neuropathol 113: 187–194
Waters P et al. Aquaporin-4 antibodies in neuromyelitis optica and longitudinally-extensive transverse myelitis. Arch Neurol, in press
Lennon VA et al. (1984) Membrane array of acetylcholine receptors determines complement-dependent mononuclear phagocytosis in experimental myasthenia gravis. Fed Proc 43: 1764
Lalive PH et al. (2006) Identification of new serum autoantibodies in neuromyelitis optica using protein microarrays. Neurology 67: 176–177
Lee KH et al. (1991) Magnetic resonance imaging of the head in the diagnosis of multiple sclerosis: a prospective 2-year follow-up with comparison of clinical evaluation, evoked potentials, oligoclonal banding, and CT. Neurology 41: 657–660
Magana SM et al. (2007) NMO-IgG status in fulminant CNS inflammatory demyelinating disorders (IDD). Neurology 68: A18–A19
Acknowledgements
The work of S Jarius was supported by a fellowship from the European Neurological Society. The authors are very grateful to Professor Margaret Esiri for helpful comments on the manuscript, and to Dr Isabel Leite and Dr Saiju Jacob for allowing us to show unpublished data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
A Vincent is a consultant and patent holder for Athena Diagnostics and RSR Ltd. The other authors declared no competing interests.
Rights and permissions
About this article
Cite this article
Jarius, S., Paul, F., Franciotta, D. et al. Mechanisms of Disease: aquaporin-4 antibodies in neuromyelitis optica. Nat Rev Neurol 4, 202–214 (2008). https://doi.org/10.1038/ncpneuro0764
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpneuro0764
This article is cited by
-
Persistent virus-specific and clonally expanded antibody-secreting cells respond to induced self-antigen in the CNS
Acta Neuropathologica (2023)
-
Inositol 1,4,5-trisphosphate receptor type 1 autoantibody (ITPR1-IgG/anti-Sj)-associated autoimmune cerebellar ataxia, encephalitis and peripheral neuropathy: review of the literature
Journal of Neuroinflammation (2022)
-
Herpes zoster preceding neuromyelitis optica spectrum disorder: casual or causal relationship? A systematic literature review
Journal of NeuroVirology (2022)
-
Correlations among disability, anti-AQP4 antibody status and prognosis in the spinal cord involved patients with NMOSD
BMC Neurology (2021)
-
Aquaporin-4-Antikörper-positive-Neuromyelitis-optica-Spektrum-Erkrankungen und Myelinoligodendrozytenglykoprotein-Antikörper-assoziierte Enzephalomyelitis. Eine Kurzübersicht
Der Nervenarzt (2021)