Key Points
-
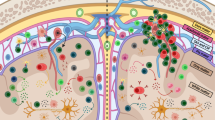
The roles of lymphocytes in multiple sclerosis (MS) pathophysiology are well known, but myeloid cells, including monocytes, macrophages, microglia and dendritic cells, are also important
-
Interactions between lymphocytes and myeloid cells exacerbate injurious processes
-
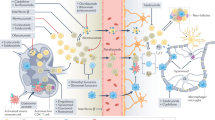
Myeloid cells are not the main targets of immunomodulators that are used to treat MS, but are still affected by them in disease-relevant ways
-
The actions of MS immunomodulators on myeloid cells contribute to the clinical efficacy of these therapeutic approaches
Abstract
Discussions of multiple sclerosis (MS) pathophysiology tend to focus on T cells and B cells of the adaptive immune response. The innate immune system is less commonly considered in this context, although dendritic cells, monocytes, macrophages and microglia — collectively referred to as myeloid cells — have prominent roles in MS pathogenesis. These populations of myeloid cells function as antigen-presenting cells and effector cells in neuroinflammation. Furthermore, a vicious cycle of interactions between T cells and myeloid cells exacerbates pathology. Several disease-modifying therapies are now available to treat MS, and insights into their mechanisms of action have largely focused on the adaptive immune system, but these therapies also have important effects on myeloid cells. In this Review, we discuss the evidence for the roles of myeloid cells in MS and the experimental autoimmune encephalomyelitis model of MS, and consider how interactions between myeloid cells and T cells and/or B cells promote MS pathology. Finally, we discuss the direct and indirect effects of existing MS medications on myeloid cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lucchinetti, C. et al. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann. Neurol. 47, 707–717 (2000).
Prineas, J. W. et al. Immunopathology of secondary-progressive multiple sclerosis. Ann. Neurol. 50, 646–657 (2001).
Howell, O. W. et al. Activated microglia mediate axoglial disruption that contributes to axonal injury in multiple sclerosis. J. Neuropathol. Exp. Neurol. 69, 1017–1033 (2010).
Strachan-Whaley, M., Rivest, S. & Yong, V. W. Interactions between microglia and T cells in multiple sclerosis pathobiology. J. Interferon Cytokine Res. 34, 615–622 (2014).
Geissmann, F. et al. Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661 (2010). An excellent review on the types and origins of myeloid cells.
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010). Seminal work that revealed the origin and development of microglia.
Prinz, M., Tay, T. L., Wolf, Y. & Jung, S. Microglia: unique and common features with other tissue macrophages. Acta Neuropathol. 128, 319–331 (2014).
Kierdorf, K. et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 16, 273–280 (2013). A pivotal paper that describes the transcription factors that regulate the early development of microglia.
Schulz, C. et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90 (2012). This paper showed for the first time that microglia precursor cells are MyB-independent, differentiating them from cells that are generated from haematopoietic stem cells.
Gomez Perdiguero, E. et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518, 547–551 (2015).
Prinz, M., Priller, J., Sisodia, S. S. & Ransohoff, R. M. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat. Neurosci. 14, 1227–1235 (2011).
Gordon, S. & Taylor, P. R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 (2005).
Delneste, Y. et al. Interferon-γ switches monocyte differentiation from dendritic cells to macrophages. Blood 101, 143–150 (2003).
Sorokin, L. The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 10, 712–723 (2010).
Agrawal, S. M. et al. Extracellular matrix metalloproteinase inducer shows active perivascular cuffs in multiple sclerosis. Brain 136, 1760–1777 (2013).
Tran, E. H., Hoekstra, K., van Rooijen, N., Dijkstra, C. D. & Owens, T. Immune invasion of the central nervous system parenchyma and experimental allergic encephalomyelitis, but not leukocyte extravasation from blood, are prevented in macrophage-depleted mice. J. Immunol. 161, 3767–3775 (1998).
Gordon, S. & Martinez, F. O. Alternative activation of macrophages: mechanism and functions. Immunity 32, 593–604 (2010).
Murray, P. J. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 (2014). This review calls for consensus in the nomenclature to describe the various activation states of macrophages.
Xue, J. et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40, 274–288 (2014). Through RNA profiling in macrophages exposed to different activators, this paper reveals a broad spectrum of activated macrophages.
Martinez, F. O. & Gordon, S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6, 13 (2014).
Agrawal, S. M., Silva, C., Tourtellotte, W. W. & Yong, V. W. EMMPRIN: a novel regulator of leukocyte transmigration into the CNS in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Neurosci. 31, 669–677 (2011).
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005). A seminal paper in which real-time videomicroscopy was used to show for the first time that microglial processes constantly move and survey their environment in vivo.
Czeh, M., Gressens, P. & Kaindl, A. M. The yin and yang of microglia. Dev. Neurosci. 33, 199–209 (2011).
Saijo, K. & Glass, C. K. Microglial cell origin and phenotypes in health and disease. Nat. Rev. Immunol. 11, 775–787 (2011).
Rawji, K. S. & Yong, V. W. The benefits and detriments of macrophages/microglia in models of multiple sclerosis. Clin. Dev. Immunol. 2013, 948976 (2013).
Casano, A. M. & Peri, F. Microglia: multitasking specialists of the brain. Dev. Cell 32, 469–477 (2015).
Reizis, B., Bunin, A., Ghosh, H. S., Lewis, K. L. & Sisirak, V. Plasmacytoid dendritic cells: recent progress and open questions. Annu. Rev. Immunol. 29, 163–183 (2011).
Miller, S. D., McMahon, E. J., Schreiner, B. & Bailey, S. L. Antigen presentation in the CNS by myeloid dendritic cells drives progression of relapsing experimental autoimmune encephalomyelitis. Ann. N. Y. Acad. Sci. 1103, 179–191 (2007).
Duraes, F. V. et al. pDC therapy induces recovery from EAE by recruiting endogenous pDC to sites of CNS inflammation. J. Autoimmun. 67, 8–18 (2016).
King, I. L., Dickendesher, T. L. & Segal, B. M. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood 113, 3190–3197 (2009).
Mildner, A. et al. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain 132, 2487–2500 (2009).
Mishra, M. K., Wang, J., Silva, C., Mack, M. & Yong, V. W. Kinetics of proinflammatory monocytes in a model of multiple sclerosis and its perturbation by laquinimod. Am. J. Pathol. 181, 642–651 (2012).
Ajami, B., Bennett, J. L., Krieger, C., McNagny, K. M. & Rossi, F. M. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 14, 1142–1149 (2011). A landmark paper showing that the microglial population is not replenished from peripheral immune cells in adulthood.
Agrawal, S. et al. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J. Exp. Med. 203, 1007–1019 (2006).
Yong, V. W., Power, C., Forsyth, P. & Edwards, D. R. Metalloproteinases in biology and pathology of the nervous system. Nat. Rev. Neurosci. 2, 502–511 (2001).
Nuttall, R. K. et al. Metalloproteinases are enriched in microglia compared with leukocytes and they regulate cytokine levels in activated microglia. Glia 55, 516–526 (2007).
Brosnan, C. F., Bornstein, M. B. & Bloom, B. R. The effects of macrophage depletion on the clinical and pathologic expression of experimental allergic encephalomyelitis. J. Immunol. 126, 614–620 (1981).
Huitinga, I., van Rooijen, N., de Groot, C. J., Uitdehaag, B. M. & Dijkstra, C. D. Suppression of experimental allergic encephalomyelitis in Lewis rats after elimination of macrophages. J. Exp. Med. 172, 1025–1033 (1990).
Bauer, J. et al. The role of macrophages, perivascular cells, and microglial cells in the pathogenesis of experimental autoimmune encephalomyelitis. Glia 15, 437–446 (1995).
Sloka, S., Metz, L. M., Hader, W., Starreveld, Y. & Yong, V. W. Reduction of microglial activity in a model of multiple sclerosis by dipyridamole. J. Neuroinflamm. 10, 89 (2013).
Mikita, J. et al. Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis. Amelioration of clinical status by M2 activated monocyte administration. Mult. Scler. 17, 2–15 (2011).
Weber, M. S. et al. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat. Med. 13, 935–943 (2007). This manuscript highlights the generation of regulatory myeloid cells by glatiramer acetate treatment, leading to the production of T H 2 cells commonly associated with this medication.
Benveniste, E. N. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J. Mol. Med. (Berl.) 75, 165–173 (1997).
Sosa, R. A., Murphey, C., Robinson, R. R. & Forsthuber, T. G. IFN-γ ameliorates autoimmune encephalomyelitis by limiting myelin lipid peroxidation. Proc. Natl Acad. Sci. USA 112, E5038–E5047 (2015).
Nikic, I. et al. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat. Med. 17, 495–499 (2011).
Mossakowski, A. A. et al. Tracking CNS and systemic sources of oxidative stress during the course of chronic neuroinflammation. Acta Neuropathol. 130, 799–814 (2015).
van Horssen, J., Witte, M. E., Schreibelt, G. & de Vries, H. E. Radical changes in multiple sclerosis pathogenesis. Biochim. Biophys. Acta 1812, 141–150 (2011).
Yamasaki, R. et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J. Exp. Med. 211, 1533–1549 (2014). This important paper revealed the different roles of microglia and macrophages in the experimental autoimmune encephalitis brain.
Brosnan, C. F., Sacks, H. J., Goldschmidt, R. C., Goldmuntz, E. A. & Norton, W. T. Prazosin treatment during the effector stage of disease suppresses experimental autoimmune encephalomyelitis in the Lewis rat. J. Immunol. 137, 3451–3456 (1986).
Shaked, I. et al. Transcription factor Nr4a1 couples sympathetic and inflammatory cues in CNS-recruited macrophages to limit neuroinflammation. Nat. Immunol. 16, 1228–1234 (2015). An important paper that shows that noradrenaline from macrophages helps promote the migration of leukocytes into the CNS, thereby linking stress and neuroinflammation.
Ponomarev, E. D., Shriver, L. P., Maresz, K. & Dittel, B. N. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J. Neurosci. Res. 81, 374–389 (2005).
Rasmussen, S. et al. Persistent activation of microglia is associated with neuronal dysfunction of callosal projecting pathways and multiple sclerosis-like lesions in relapsing–remitting experimental autoimmune encephalomyelitis. Brain 130, 2816–2829 (2007).
Takeuchi, H. et al. Tumor necrosis factor-α induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J. Biol. Chem. 281, 21362–21368 (2006).
Aloisi, F., Ria, F., Penna, G. & Adorini, L. Microglia are more efficient than astrocytes in antigen processing and in Th1 but not Th2 cell activation. J. Immunol. 160, 4671–4680 (1998).
Aloisi, F. et al. Relative efficiency of microglia, astrocytes, dendritic cells and B cells in naive CD4+ T cell priming and Th1/Th2 cell restimulation. Eur. J. Immunol. 29, 2705–2714 (1999).
Davalos, D. et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat. Commun. 3, 1227 (2012).
Heppner, F. L. et al. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat. Med. 11, 146–152 (2005).
Goldmann, T. et al. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat. Neurosci. 16, 1618–1626 (2013).
Neumann, J. et al. Microglia cells protect neurons by direct engulfment of invading neutrophil granulocytes: a new mechanism of CNS immune privilege. J. Neurosci. 28, 5965–5975 (2008).
Chen, Z. et al. Microglial displacement of inhibitory synapses provides neuroprotection in the adult brain. Nat. Commun. 5, 4486 (2014).
Ferguson, B., Matyszak, M. K., Esiri, M. M. & Perry, V. H. Axonal damage in acute multiple sclerosis lesions. Brain 120, 393–399 (1997).
Politis, M. et al. Increased PK11195 PET binding in the cortex of patients with MS correlates with disability. Neurology 79, 523–530 (2012). An important paper that describes the detection of activated microglia and macrophages through PET imaging in people with multiple sclerosis.
Kutzelnigg, A. et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128, 2705–2712 (2005).
van Noort, J. M. et al. Preactive multiple sclerosis lesions offer novel clues for neuroprotective therapeutic strategies. CNS Neurol. Disord. Drug Targets 10, 68–81 (2011).
Vogel, D. Y. et al. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J. Neuroinflamm. 10, 35 (2013).
Kooi, E. J., Strijbis, E. M., van der Valk, P. & Geurts, J. J. Heterogeneity of cortical lesions in multiple sclerosis: clinical and pathologic implications. Neurology 79, 1369–1376 (2012).
Singh, S. et al. Microglial nodules in early multiple sclerosis white matter are associated with degenerating axons. Acta Neuropathol. 125, 595–608 (2013).
Peferoen, L. A. et al. Activation status of human microglia is dependent on lesion formation stage and remyelination in multiple sclerosis. J. Neuropathol. Exp. Neurol. 74, 48–63 (2015).
Kouwenhoven, M., Teleshova, N., Ozenci, V., Press, R. & Link, H. Monocytes in multiple sclerosis: phenotype and cytokine profile. J. Neuroimmunol. 112, 197–205 (2001).
Huang, Y. M. et al. Multiple sclerosis is associated with high levels of circulating dendritic cells secreting pro-inflammatory cytokines. J. Neuroimmunol. 99, 82–90 (1999).
Makhlouf, K., Weiner, H. L. & Khoury, S. J. Increased percentage of IL-12+ monocytes in the blood correlates with the presence of active MRI lesions in MS. J. Neuroimmunol. 119, 145–149 (2001).
Waschbisch, A. et al. Pivotal role for CD16+ monocytes in immune surveillance of the central nervous system. J. Immunol. 196, 1558–1567 (2016).
Kivisakk, P. et al. Localizing central nervous system immune surveillance: meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann. Neurol. 65, 457–469 (2009).
Croxford, A. L. et al. The cytokine GM-CSF drives the inflammatory signature of CCR2+ monocytes and licenses autoimmunity. Immunity 43, 502–514 (2015). A key paper that links T H 17 cells and the production of GM-CSF with the downstream generation of proinflammatory monocytes that mediate CNS inflammation.
Codarri, L., Greter, M. & Becher, B. Communication between pathogenic T cells and myeloid cells in neuroinflammatory disease. Trends Immunol. 34, 114–119 (2013).
Ponomarev, E. D. et al. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J. Immunol. 178, 39–48 (2007).
Codarri, L. et al. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 12, 560–567 (2011).
El-Behi, M. et al. The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 12, 568–575 (2011).
Croxford, A. L., Spath, S. & Becher, B. GM-CSF in neuroinflammation: licensing myeloid cells for tissue damage. Trends Immunol. 36, 651–662 (2015). An excellent review that discusses the critical role of myeloid cells in tissue damage in the CNS.
Bruck, W. et al. Therapeutic decisions in multiple sclerosis: moving beyond efficacy. JAMA Neurol. 70, 1315–1324 (2013).
Stuve, O. et al. Pharmacological treatment of early multiple sclerosis. Drugs 68, 73–83 (2008).
Biber, K., Moller, T., Boddeke, E. & Prinz, M. Central nervous system myeloid cells as drug targets: current status and translational challenges. Nat. Rev. Drug Discov. 15, 110–124 (2016). This comprehensive review discusses the effector functions of myeloid cells and the approaches to targeting the proinflammatory activity of myeloid cells.
Dhib-Jalbut, S. & Marks, S. Interferon-beta mechanisms of action in multiple sclerosis. Neurology 74, S17–S24 (2010).
Marckmann, S. et al. Interferon-β up-regulates the expression of co-stimulatory molecules CD80, CD86 and CD40 on monocytes: significance for treatment of multiple sclerosis. Clin. Exp. Immunol. 138, 499–506 (2004).
Liu, Z., Pelfrey, C. M., Cotleur, A., Lee, J. C. & Rudick, R. A. Immunomodulatory effects of interferon beta-1a in multiple sclerosis. J. Neuroimmunol. 112, 153–162 (2001).
Ramgolam, V. S., Sha, Y., Jin, J., Zhang, X. & Markovic-Plese, S. IFN-β inhibits human Th17 cell differentiation. J. Immunol. 183, 5418–5427 (2009).
Yen, J. H., Kong, W. & Ganea, D. IFN-β inhibits dendritic cell migration through STAT-1-mediated transcriptional suppression of CCR7 and matrix metalloproteinase 9. J. Immunol. 184, 3478–3486 (2010). An important paper that shows the direct effect of IFN-β on dendritic cells.
Galboiz, Y., Shapiro, S., Lahat, N. & Miller, A. Modulation of monocytes matrix metalloproteinase-2, MT1-MMP and TIMP-2 by interferon-γ and -β: implications to multiple sclerosis. J. Neuroimmunol. 131, 191–200 (2002).
Schreiner, B. et al. Interferon-β enhances monocyte and dendritic cell expression of B7-H1 (PD-L1), a strong inhibitor of autologous T-cell activation: relevance for the immune modulatory effect in multiple sclerosis. J. Neuroimmunol. 155, 172–182 (2004).
Comabella, M. et al. A type I interferon signature in monocytes is associated with poor response to interferon-β in multiple sclerosis. Brain 132, 3353–3365 (2009).
Yen, J. H. & Ganea, D. Interferon β induces mature dendritic cell apoptosis through caspase-11/caspase-3 activation. Blood 114, 1344–1354 (2009).
Guo, B., Chang, E. Y. & Cheng, G. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J. Clin. Invest. 118, 1680–1690 (2008).
Prinz, M. et al. Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity 28, 675–686 (2008). A key paper that implicates myeloid cells as important cellular targets of IFN-β signalling to ameliorate neuroinflammation.
Hussien, Y., Sanna, A., Soderstrom, M., Link, H. & Huang, Y. M. Multiple sclerosis: expression of CD1a and production of IL-12p70 and IFN-γ by blood mononuclear cells in patients on combination therapy with IFN-β and glatiramer acetate compared to monotherapy with IFN-β. Mult. Scler. 10, 16–25 (2004).
Lucas, M. et al. Regulation by interferon β-1a of reactive oxygen metabolites production by lymphocytes and monocytes and serum sulfhydryls in relapsing multiple sclerosis patients. Neurochem. Int. 42, 67–71 (2003).
Hamamcioglu, K. & Reder, A. T. Interferon-β regulates cytokines and BDNF: greater effect in relapsing than in progressive multiple sclerosis. Mult. Scler. 13, 459–470 (2007).
Waschbisch, A. et al. Interferon beta and vitamin D synergize to induce immunoregulatory receptors on peripheral blood monocytes of multiple sclerosis patients. PLoS ONE 9, e115488 (2014).
Weber, M. S. et al. Multiple sclerosis: glatiramer acetate inhibits monocyte reactivity in vitro and in vivo. Brain 127, 1370–1378 (2004).
Kim, H. J. et al. Type 2 monocyte and microglia differentiation mediated by glatiramer acetate therapy in patients with multiple sclerosis. J. Immunol. 172, 7144–7153 (2004). One of the first papers to describe the generation of regulatory myeloid cells by glatiramer acetate.
Sellebjerg, F. et al. Dendritic cell, monocyte and T cell activation and response to glatiramer acetate in multiple sclerosis. Mult. Scler. 19, 179–187 (2013).
Iarlori, C. et al. Reduction of free radicals in multiple sclerosis: effect of glatiramer acetate (Copaxone). Mult. Scler. 14, 739–748 (2008).
Pul, R. et al. Glatiramer acetate modulates TNF-α and IL-10 secretion in microglia and promotes their phagocytic activity. J. Neuroimmune Pharmacol. 6, 381–388 (2011).
Pul, R. et al. Glatiramer acetate increases phagocytic activity of human monocytes in vitro and in multiple sclerosis patients. PLoS ONE 7, e51867 (2012).
Burger, D. et al. Glatiramer acetate increases IL-1 receptor antagonist but decreases T cell-induced IL-1β in human monocytes and multiple sclerosis. Proc. Natl Acad. Sci. USA 106, 4355–4359 (2009). This study shows that human monocytes are responsive to glatiramer acetate and that they increase their regulatory properties as a result.
Ayers, C. L. et al. Modulation of immune function occurs within hours of therapy initiation for multiple sclerosis. Clin. Immunol. 147, 105–119 (2013).
Ratchford, J. N. et al. Decreased microglial activation in MS patients treated with glatiramer acetate. J. Neurol. 259, 1199–1205 (2012).
Chun, J. & Hartung, H. P. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin. Neuropharmacol. 33, 91–101 (2010).
Muller, H. et al. The immunomodulator FTY720 interferes with effector functions of human monocyte-derived dendritic cells. Eur. J. Immunol. 35, 533–545 (2005).
Awojoodu, A. O. et al. Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc. Natl Acad. Sci. USA 110, 13785–13790 (2013).
Hughes, J. E. et al. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ. Res. 102, 950–958 (2008).
Durafourt, B. A. et al. Differential responses of human microglia and blood-derived myeloid cells to FTY720. J. Neuroimmunol. 230, 10–16 (2011).
Noda, H., Takeuchi, H., Mizuno, T. & Suzumura, A. Fingolimod phosphate promotes the neuroprotective effects of microglia. J. Neuroimmunol. 256, 13–18 (2013). The study that demonstrated that microglia are altered by fingolimod exposure to generate growth factors.
Al-Jaderi, Z. & Maghazachi, A. A. Effects of vitamin D3, calcipotriol and FTY720 on the expression of surface molecules and cytolytic activities of human natural killer cells and dendritic cells. Toxins (Basel) 5, 1932–1947 (2013).
Jackson, S. J., Giovannoni, G. & Baker, D. Fingolimod modulates microglial activation to augment markers of remyelination. J. Neuroinflamm. 8, 76 (2011). This study demonstrated that microglia are affected by fingolimod, and that they become pro-reparative as a result.
Lewis, N. D. et al. Circulating monocytes are reduced by sphingosine-1-phosphate receptor modulators independently of S1P3. J. Immunol. 190, 3533–3540 (2013).
Luessi, F. et al. FTY720 (fingolimod) treatment tips the balance towards less immunogenic antigen-presenting cells in patients with multiple sclerosis. Mult. Scler. 21, 1811–1822 (2015).
Michell-Robinson, M. A. et al. Effects of fumarates on circulating and CNS myeloid cells in multiple sclerosis. Ann. Clin. Transl. Neurol. 3, 27–41 (2016). This study showed that monocytes from patients with multiple sclerosis who were treated with dimethylfumarate had reduced expression of the proinflammatory microRNA miR-155.
Linker, R. A. et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 134, 678–692 (2011).
Peng, H. et al. Dimethyl fumarate inhibits dendritic cell maturation via nuclear factor κB (NF-κB) and extracellular signal-regulated kinase 1 and 2 (ERK1/2) and mitogen stress-activated kinase 1 (MSK1) signaling. J. Biol. Chem. 287, 28017–28026 (2012).
Wilms, H. et al. Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1β, TNF-α and IL-6 in an in-vitro model of brain inflammation. J. Neuroinflamm. 7, 30 (2010).
Cross, S. A. et al. Dimethyl fumarate, an immune modulator and inducer of the antioxidant response, suppresses HIV replication and macrophage-mediated neurotoxicity: a novel candidate for HIV neuroprotection. J. Immunol. 187, 5015–5025 (2011).
Schilling, S., Goelz, S., Linker, R., Luehder, F. & Gold, R. Fumaric acid esters are effective in chronic experimental autoimmune encephalomyelitis and suppress macrophage infiltration. Clin. Exp. Immunol. 145, 101–107 (2006).
Spencer, C. M., Crabtree-Hartman, E. C., Lehmann-Horn, K., Cree, B. A. & Zamvil, S. S. Reduction of CD8+ T lymphocytes in multiple sclerosis patients treated with dimethyl fumarate. Neurol. Neuroimmunol. Neuroinflamm. 2, e76 (2015).
Ghoreschi, K. et al. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J. Exp. Med. 208, 2291–2303 (2011). This manuscript suggested for the first time that dimethylfumarate has an effect on the generation of regulatory dendritic cells.
Tanasescu, R., Evangelou, N. & Constantinescu, C. S. Role of oral teriflunomide in the management of multiple sclerosis. Neuropsychiatr. Dis. Treat. 9, 539–553 (2013).
Korn, T., Magnus, T., Toyka, K. & Jung, S. Modulation of effector cell functions in experimental autoimmune encephalomyelitis by leflunomide — mechanisms independent of pyrimidine depletion. J. Leukoc. Biol. 76, 950–960 (2004).
Ringheim, G. E. et al. Teriflunomide attenuates immunopathological changes in the dark agouti rat model of experimental autoimmune encephalomyelitis. Front. Neurol. 4, 169 (2013).
Li, L. et al. The effects of teriflunomide on lymphocyte subpopulations in human peripheral blood mononuclear cells in vitro. J. Neuroimmunol. 265, 82–90 (2013).
Mishra, M. K. et al. Laquinimod reduces neuroaxonal injury through inhibiting microglial activation. Ann. Clin. Transl. Neurol. 1, 409–422 (2014). A manuscript that describes that microglial activation is normalized by laquinimod.
Schulze-Topphoff, U. et al. Laquinimod, a quinoline-3-carboxamide, induces type II myeloid cells that modulate central nervous system autoimmunity. PLoS ONE 7, e33797 (2012).
Jolivel, V. et al. Modulation of dendritic cell properties by laquinimod as a mechanism for modulating multiple sclerosis. Brain 136, 1048–1066 (2013).
Thone, J. et al. Modulation of autoimmune demyelination by laquinimod via induction of brain-derived neurotrophic factor. Am. J. Pathol. 180, 267–274 (2012).
Lund, B. T. et al. Assessment of changes in immune measures of multiple sclerosis patients treated with laquinimod. J. Neuroimmunol. 263, 108–115 (2013).
Ali, R., Nicholas, R. S. & Muraro, P. A. Drugs in development for relapsing multiple sclerosis. Drugs 73, 625–650 (2013).
Planas, R., Jelcic, I., Schippling, S., Martin, R. & Sospedra, M. Natalizumab treatment perturbs memory- and marginal zone-like B-cell homing in secondary lymphoid organs in multiple sclerosis. Eur. J. Immunol. 42, 790–798 (2012).
Jones, J. L. & Coles, A. J. Mode of action and clinical studies with alemtuzumab. Exp. Neurol. 262, 37–43 (2014).
Coles, A. J. et al. The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J. Neurol. 253, 98–108 (2006).
Boster, A., Ankeny, D. P. & Racke, M. K. The potential role of B cell-targeted therapies in multiple sclerosis. Drugs 70, 2343–2356 (2010).
Li, R. et al. Proinflammatory GM-CSF-producing B cells in multiple sclerosis and B cell depletion therapy. Sci. Transl. Med. 7, 310ra166 (2015). An excellent paper that showed that the depletion of B cells in patients with multiple sclerosis led to decreases in proinflammatory myeloid responses.
Kausar, F. et al. Ocrelizumab: a step forward in the evolution of B-cell therapy. Expert. Opin. Biol. Ther. 9, 889–895 (2009).
Schweingruber, N., Reichardt, S. D., Luhder, F. & Reichardt, H. M. Mechanisms of glucocorticoids in the control of neuroinflammation. J. Neuroendocrinol. 24, 174–182 (2012).
Joyce, D. A., Steer, J. H. & Abraham, L. J. Glucocorticoid modulation of human monocyte/macrophage function: control of TNF-α secretion. Inflamm. Res. 46, 447–451 (1997).
Parrillo, J. E. & Fauci, A. S. Mechanisms of glucocorticoid action on immune processes. Annu. Rev. Pharmacol. Toxicol. 19, 179–201 (1979).
DeKruyff, R. H., Fang, Y. & Umetsu, D. T. Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. J. Immunol. 160, 2231–2237 (1998).
Schweingruber, N. et al. Liposomal encapsulation of glucocorticoids alters their mode of action in the treatment of experimental autoimmune encephalomyelitis. J. Immunol. 187, 4310–4318 (2011).
Lee, D. H. et al. Glutathione PEGylated liposomal methylprednisolone (2B3-201) attenuates CNS inflammation and degeneration in murine myelin oligodendrocyte glycoprotein induced experimental autoimmune encephalomyelitis. J. Neuroimmunol. 274, 96–101 (2014).
Frisullo, G. et al. Glucocorticoid treatment reduces T-bet and pSTAT1 expression in mononuclear cells from relapsing remitting multiple sclerosis patients. Clin. Immunol. 124, 284–293 (2007).
Krystyna, M. S. et al. Changes in circulating dendritic cells and B-cells in patients with multiple sclerosis relapse during corticosteroid therapy. J. Neuroimmunol. 207, 107–110 (2009).
Gayo, A. et al. Glucocorticoids increase IL-10 expression in multiple sclerosis patients with acute relapse. J. Neuroimmunol. 85, 122–130 (1998).
Wee Yong, V. Inflammation in neurological disorders: a help or a hindrance? Neuroscientist 16, 408–420 (2010).
Acknowledgements
The authors' research is supported by grants from the Canadian Institutes of Health Research, the Alberta Innovates–Health Solutions CRIO Team programme, and the Multiple Sclerosis Society of Canada.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of the manuscript.
Corresponding author
Ethics declarations
Competing interests
V.W.Y. acknowledges previous unrestricted operating grant funding from EMD-Serono, Novartis and Teva Pharmaceuticals, and previous and current unrestricted educational grants from Biogen-Idec, EMD-Serono, Genyzme, Novartis, Roche and Teva Neuroscience. V.W.Y. has received honoraria for seminar presentations from Biogen-Idec, Genzyme, Novartis and Teva Neuroscience. M.K.M. declares no competing interests.
Rights and permissions
About this article
Cite this article
Mishra, M., Yong, V. Myeloid cells — targets of medication in multiple sclerosis. Nat Rev Neurol 12, 539–551 (2016). https://doi.org/10.1038/nrneurol.2016.110
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2016.110
This article is cited by
-
Impact of disease-modifying therapy on dendritic cells and exploring their immunotherapeutic potential in multiple sclerosis
Journal of Neuroinflammation (2022)
-
SLAMF7 modulates B cells and adaptive immunity to regulate susceptibility to CNS autoimmunity
Journal of Neuroinflammation (2022)
-
Microglia and monocytes in inflammatory CNS disease: integrating phenotype and function
Acta Neuropathologica (2022)
-
ADAMTS13 ameliorates inflammatory responses in experimental autoimmune encephalomyelitis
Journal of Neuroinflammation (2020)
-
The neutrophil-to-lymphocyte and monocyte-to-lymphocyte ratios are independently associated with neurological disability and brain atrophy in multiple sclerosis
BMC Neurology (2019)