Key Points
-
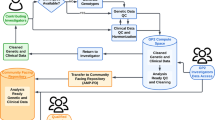
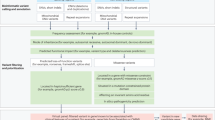
Consortium for Clinical Investigations of Neurological Channelopathies (CINCH) and Clinical Research Consortium for Spinocerebellar Ataxias (CRC-SCA) are multicentre networks that engage patients who have rare neurological channelopathies with investigators, as well as advocacy groups
-
Shared goals of the networks are to improve diagnosis, characterize the natural history, understand disease mechanisms, develop outcome measures that can be assessed in clinical trials, and devise novel treatment
-
Patients, advocacy groups and investigators are partners in network activities, including teleconferences for study organization and training, steering committee meetings, and annual face-to-face research workshops and scientific symposia
-
CINCH and CRC-SCA have invested in mentoring of junior investigators to pursue patient-oriented research on rare channelopathies
-
The two networks have created and maintained patient registries, stratified patients on the basis of genetic characteristics, collected longitudinal clinical data to identify disease-relevant outcome measures, and conducted Phase I and II trials
-
The two networks illustrate the collaborative approach that is necessary for the growing list of rare neurological channelopathies in need of treatment and further investigation
Abstract
Each of the thousands of rare neurological diseases requires a widely distributed network of centres, investigators and patients, so as to foster multidisciplinary investigations and involve sufficient numbers of patients in the discovery of disease pathogenesis and novel treatment. In this Review, we highlight the value of this collaborative approach in patient-oriented research into rare neurological channelopathies. Two networks, the Consortium for Clinical Investigations of Neurological Channelopathies (CINCH) and the Clinical Research Consortium for Studies of Cerebellar Ataxias (CRC-SCA), provide a link between patients with rare channelopathies and investigators who are studying disease pathogenesis and developing novel treatments. Interactions between patients, researchers and advocacy groups promote shared agendas that benefit patient education and recruitment, research collaboration and funding, and training and mentoring of junior investigators who are attracted to the study of the diseases that provide the focus for the two networks. Here, we discuss how linkage of national and international centres has enabled recruitment of study participants, provided opportunities for novel studies of pathogenesis, and facilitated successful clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Griggs, R. C. et al. Clinical research for rare disease: opportunities, challenges, and solutions. Mol. Genet. Metab. 96, 20–26 (2009).
Murphy, S. M., Puwanant, A. & Griggs, R. C. Unintended effects of orphan product designation for rare neurological diseases. Ann. Neurol. 72, 481–490 (2012).
Venance, S. L., Herr, B. E. & Griggs, R. C. Challenges in the design and conduct of therapeutic trials in channel disorders. Neurotherapeutics 4, 199–204 (2007).
Cleland, J. C. & Griggs, R. C. Treatment of neuromuscular channelopathies: current concepts and future prospects. Neurotherapeutics 5, 607–612 (2008).
Venance, S. L. et al. The primary periodic paralyses: diagnosis, pathogenesis and treatment. Brain 129, 8–17 (2006).
Ptácek, L. J. et al. Identification of a mutation in the gene causing hyperkalemic periodic paralysis. Cell 67, 1021–1027 (1991).
Ptácek, L. J. et al. Dihydropyridine receptor mutations cause hypokalemic periodic paralysis. Cell 77, 863–868 (1994).
Plaster, N. M. et al. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen's syndrome. Cell 105, 511–519 (2001).
Kokunai, Y. et al. A Kir3.4 mutation causes Andersen–Tawil syndrome by an inhibitory effect on Kir2.1. Neurology 82, 1058–1064 (2014).
Koch, M. C. et al. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science 257, 797–800 (1992).
Ptácek, L. J. et al. Mutations in an S4 segment of the adult skeletal muscle sodium channel cause paramyotonia congenita. Neuron 8, 891–897 (1992).
Brook, J. D. et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 68, 799–808 (1992).
Mankodi, A. et al. Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy. Mol. Cell 10, 35–44 (2002).
Charlet-B., N. et al. Loss of the muscle-specific chloride channel in type 1 myotonic dystrophy due to misregulated alternative splicing. Mol. Cell 10, 45–53 (2002).
Chen, W. et al. Haploinsuffciency for Znf9 in Znf9+/− mice is associated with multiorgan abnormalities resembling myotonic dystrophy. J. Mol. Biol. 368, 8–17 (2007).
Jen, J. C. et al. Primary episodic ataxias: diagnosis, pathogenesis and treatment. Brain 130, 2484–2493 (2007).
Conroy, J. et al. A novel locus for episodic ataxia: UBR4 the likely candidate. Eur. J. Hum. Genet. 22, 505–510 (2014).
Julien, J. et al. Sporadic late onset paroxysmal cerebellar ataxia in four unrelated patients: a new disease? J. Neurol. 248, 209–214 (2001).
Damak, M. et al. Late onset hereditary episodic ataxia. J. Neurol. Neurosurg. Psychiatry 80, 566–568 (2009).
Browne, D. L. et al. Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nat. Genet. 8, 136–140 (1994).
Ophoff, R. A. et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell 87, 543–552 (1996).
Harding, A. E. The clinical features and classification of the late onset autosomal dominant cerebellar ataxias: a study of 11 families, including descendants of 'the Drew family of Walworth'. Brain 105, 1–28 (1982).
Zhuchenko, O. et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the α1A-voltage-dependent calcium channel. Nat. Genet. 15, 62–69 (1997).
Waters, M. F. et al. Mutations in voltage-gated potassium channel KCNC3 cause degenerative and developmental central nervous system phenotypes. Nat. Genet. 38, 447–451 (2006).
Duarri, A. et al. Mutations in potassium channel kcnd3 cause spinocerebellar ataxia type 19. Ann. Neurol. 72, 870–880 (2012).
Lee, Y. C. et al. Mutations in KCND3 cause spinocerebellar ataxia type 22. Ann. Neurol. 72, 859–869 (2012).
van de Leemput, J. et al. Deletion at ITPR1 underlies ataxia in mice and spinocerebellar ataxia 15 in humans. PLoS Genet. 3, e108 (2007).
Huang, L. et al. Missense mutations in ITPR1 cause autosomal dominant congenital nonprogressive spinocerebellar ataxia. Orphanet J. Rare Dis. 7, 67 (2012).
Iwaki, A. et al. Heterozygous deletion of ITPR1, but not SUMF1, in spinocerebellar ataxia type 16. J. Med. Genet. 45, 32–35 (2008).
Ke, Q. et al. Rare disease centers for periodic paralysis: China versus the United States and United Kingdom. Muscle Nerve 49, 171–174 (2014).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–423 (2015).
Subramony, S. H. et al. Comprehensive phenotype of the p.Arg420his allelic form of spinocerebellar ataxia type 13. Cerebellum 12, 932–936 (2013).
Waters, M. F., Subramony, S. H., Advincula, J., Perlman, S. & Ashizawa, T. Oculomotor and visual axis systems sparing in spinocerebellar ataxia type 13R420H. Neurology 79, 1181–1182 (2012).
Figueroa, K. P. et al. Frequency of KCNC3 DNA variants as causes of spinocerebellar ataxia 13 (SCA13). PLoS ONE 6, e17811 (2011).
Figueroa, K. P. et al. KCNC3: phenotype, mutations, channel biophysics — a study of 260 familial ataxia patients. Hum. Mutat. 31, 191–196 (2010).
Kerem, B. et al. Identification of the cystic fibrosis gene: genetic analysis. Science 245, 1073–1080 (1989).
Graves, T. D. et al. Episodic ataxia type 1: clinical characterization, quality of life and genotype–phenotype correlation. Brain 137, 1009–1018 (2014).
Labrum, R. W. et al. Large scale calcium channel gene rearrangements in episodic ataxia and hemiplegic migraine: implications for diagnostic testing. J. Med. Genet. 46, 786–791 (2009).
Wan, J. et al. Large genomic deletions in CACNA1A cause episodic ataxia type 2. Front. Neurol. 2, 51 (2011).
Pyle, A. et al. Exome sequencing in undiagnosed inherited and sporadic ataxias. Brain 138, 276–283 (2015).
Fogel, B. L. et al. Exome sequencing in the clinical diagnosis of sporadic or familial cerebellar ataxia. JAMA Neurol. 71, 1237–1246 (2014).
Escayg, A. et al. Coding and noncoding variation of the human calcium-channel β4-subunit gene CACNB4 in patients with idiopathic generalized epilepsy and episodic ataxia. Am. J. Hum. Genet. 66, 1531–1539 (2000).
Jen, J. C., Wan, J., Palos, T. P., Howard, B. D. & Baloh, R. W. Mutation in the glutamate transporter EAAT1 causes episodic ataxia, hemiplegia, and seizures. Neurology 65, 529–534 (2005).
de Vries, B. et al. Episodic ataxia associated with EAAT1 mutation C186S affecting glutamate reuptake. Arch. Neurol. 66, 97–101 (2009).
Kerber, K. A., Jen, J. C., Lee, H., Nelson, S. F. & Baloh, R. W. A new episodic ataxia syndrome with linkage to chromosome 19q13. Arch. Neurol. 64, 749–752 (2007).
Jen, J. C., Lee, H., Cha, Y. H., Nelson, S. F. & Baloh, R. W. Genetic heterogeneity of autosomal dominant nonprogressive congenital ataxia. Neurology 67, 1704–1706 (2006).
Cha, Y. H. et al. Episodic vertical oscillopsia with progressive gait ataxia: clinical description of a new episodic syndrome and evidence of linkage to chromosome 13q. J. Neurol. Neurosurg. Psychiatry 78, 1273–1275 (2007).
Silva, G. S., Farrell, S., Shandra, E., Viswanathan, A. & Schwamm, L. H. The status of telestroke in the United States: a survey of currently active stroke telemedicine programs. Stroke 43, 2078–2085 (2012).
Wechsler, L. R. et al. Teleneurology applications: report of the Telemedicine Work Group of the American Academy of Neurology. Neurology 80, 670–676 (2013).
Guyatt, G. et al. Determining optimal therapy — randomized trials in individual patients. N. Engl. J. Med. 314, 889–892 (1986).
Duan, N., Kravitz, R. L. & Schmid, C. H. Single-patient (n-of-1) trials: a pragmatic clinical decision methodology for patient-centered comparative effectiveness research. J. Clin. Epidemiol. 66, S21–S28 (2013).
Statland, J. M. et al. Mexiletine for symptoms and signs of myotonia in nondystrophic myotonia: a randomized controlled trial. JAMA 308, 1357–1365 (2012).
Hoffman, E. P. & Kaminski, H. J. Mexiletine for treatment of myotonia: a trial triumph for rare disease networks. JAMA 308, 1377–1378 (2012).
Tawil, R. et al. Randomized trials of dichlorphenamide in the periodic paralyses. Working Group on Periodic Paralysis. Ann. Neurol. 47, 46–53 (2000).
Ashizawa, T. et al. Clinical characteristics of patients with spinocerebellar ataxias 1, 2, 3 and 6 in the US; a prospective observational study. Orphanet J. Rare Dis. 8, 177 (2013).
Schmitz-Hubsch, T. et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 66, 1717–1720 (2006).
Jacobi, H. et al. The natural history of spinocerebellar ataxia type 1, 2, 3, and 6: a 2-year follow-up study. Neurology 77, 1035–1041 (2011).
Tezenas du Montcel, S. et al. Modulation of the age at onset in spinocerebellar ataxia by CAG tracts in various genes. Brain 137, 2444–2455 (2014).
Shi, Y. et al. High serum GFAP levels in SCA3/MJD may not correlate with disease progression. Cerebellum 14, 677–681 (2015).
Teive, H. A. et al. Spinocerebellar ataxias: genotype–phenotype correlations in 104 Brazilian families. Clinics (Sao Paulo) 67, 443–449 (2012).
Matthews, E. et al. The non-dystrophic myotonias: molecular pathogenesis, diagnosis and treatment. Brain 133, 9–22 (2010).
Statland, J. M. et al. An interactive voice response diary for patients with non-dystrophic myotonia. Muscle Nerve 44, 30–35 (2011).
Trivedi, J. R. et al. Non-dystrophic myotonia: prospective study of objective and patient reported outcomes. Brain 136, 2189–2200 (2013).
Stunnenberg, B. C. et al. Combined N-of-1 trials to investigate mexiletine in non-dystrophic myotonia using a Bayesian approach; study rationale and protocol. BMC Neurol. 15, 43 (2015).
Acknowledgements
The CINCH and CRC-SCA consortia were participants of the Rare Disease Clinical Research Network supported by the Office of Rare Disease Research and the National Center for Advancing Translational Sciences of the NIH. Both consortia were supported by the National Institute of Neurological Disorders and Stroke. CINCH received support from the Muscular Dystrophy Association. CRC-SCA received support from the National Ataxia Foundation.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, made substantial contributions to discussions of the content, wrote the article, and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Jen, J., Ashizawa, T., Griggs, R. et al. Rare neurological channelopathies — networks to study patients, pathogenesis and treatment. Nat Rev Neurol 12, 195–203 (2016). https://doi.org/10.1038/nrneurol.2016.18
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2016.18
This article is cited by
-
Whole-exome sequencing in eccrine porocarcinoma indicates promising therapeutic strategies
Cancer Gene Therapy (2022)
-
Spinocerebellar ataxias: prospects and challenges for therapy development
Nature Reviews Neurology (2018)