Key Points
-
Traumatic brain injury (TBI) is caused by an external mechanical force that injures the brain parenchyma; however, most patients with mild TBI show no signs of injury on a CT scan
-
Given that mild TBI cannot usually be diagnosed objectively, accurate fluid biomarkers would be a welcome addition to the diagnostic toolbox
-
Repetitive mild TBI can cause progressive neurodegeneration, known as chronic traumatic encephalopathy (CTE); however, estimating the risk of CTE is difficult, and the condition cannot be diagnosed in living patients
-
In future, biomarkers for mild TBI, postconcussive syndrome and CTE might help us predict the risk of long-term sequelae and improve our understanding of the underlying pathophysiology
-
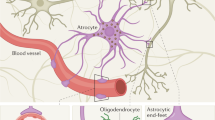
Brain specificity or brain-enhanced expression is an important characteristic of blood-based biomarkers for mild TBI and related conditions, as extracerebral sources for biomarker molecules can compromise the interpretability of the test results
-
In mild TBI, fluid biomarkers for axonal injury and astroglial activation show the greatest promise at the moment, and several other promising biomarker candidates exist
Abstract
Diagnostic and prognostic biomarkers for mild traumatic brain injury (TBI), also known as concussion, remain a major unmet clinical need. Moderate to severe TBI can be diagnosed definitively by clinical assessment and standard neuroimaging techniques that detect the gross damage to the brain parenchyma. Diagnostic tools for mild TBI are lacking and, currently, the diagnosis has to be made on clinical grounds alone, because most patients show no gross pathological changes on CT. Most patients with mild TBI recover quickly, but about 15% develop an ill-defined condition called postconcussive syndrome (PCS). Repeated concussions have been associated with a chronic neurodegenerative disorder called chronic traumatic encephalopathy (CTE), which can only currently be diagnosed post mortem. Fluid biomarkers are needed to better define and detect mild TBI and related conditions. Here, we review the literature on fluid biomarkers for neuronal, axonal, oligodendrocytic, astroglial and blood–brain barrier injury, as well as markers for neuroinflammation and metabolic dysregulation, in the context of mild TBI, PCS and CTE. We also discuss technical and standardization issues and potential pathways to advance the most promising biomarker candidates into clinical laboratory practice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Easter, J. S., Haukoos, J. S., Meehan, W. P., Novack, V. & Edlow, J. A. Will neuroimaging reveal a severe intracranial injury in this adult with minor head trauma? The Rational Clinical Examination systematic review. JAMA 314, 2672–2681 (2015).
Teasdale, G. & Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 2, 81–84 (1974).
Sandsmark, D. K. Clinical outcomes after traumatic brain injury. Curr. Neurol. Neurosci. Rep. 16, 52 (2016).
Borg, J. et al. Diagnostic procedures in mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 43, 61–75 (2004).
Levin, H. S. & Diaz-Arrastia, R. R. Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol. 14, 506–517 (2015). An extensive review on clinical aspects of mild TBI.
McCrory, P. et al. Consensus statement on concussion in sport: the Third International Conference on Concussion in Sport held in Zurich, November 2008. Phys. Sportsmed. 37, 141–159 (2009).
Mu, W., Catenaccio, E. & Lipton, M. L. Neuroimaging in blast-related mild traumatic brain injury. J. Head Trauma Rehabil. http://dx.doi.org/10.1097/HTR.0000000000000213 (2016).
Delouche, A. et al. Diffusion MRI: pitfalls, literature review and future directions of research in mild traumatic brain injury. Eur. J. Radiol. 85, 25–30 (2016).
Eisenberg, M. A., Andrea, J., Meehan, W. & Mannix, R. Time interval between concussions and symptom duration. Pediatrics 132, 8–17 (2013).
Williams, W. H., Potter, S. & Ryland, H. Mild traumatic brain injury and postconcussion syndrome: a neuropsychological perspective. J. Neurol. Neurosurg. Psychiatry 81, 1116–1122 (2010).
Broshek, D. K., De Marco, A. P. & Freeman, J. R. A review of post-concussion syndrome and psychological factors associated with concussion. Brain Inj. 29, 228–237 (2015).
Ryan, L. M. & Warden, D. L. Post concussion syndrome. Int. Rev. Psychiatry 15, 310–316 (2003).
Iverson, G. L. & Lange, R. T. Examination of “postconcussion-like” symptoms in a healthy sample. Appl. Neuropsychol. 10, 137–144 (2003).
Rathbone, A. T., Tharmaradinam, S., Jiang, S., Rathbone, M. P. & Kumbhare, D. A. A review of the neuro- and systemic inflammatory responses in post concussion symptoms: introduction of the “post-inflammatory brain syndrome” PIBS. Brain Behav. Immun. 46, 1–16 (2015).
Corsellis, J. A., Bruton, C. J. & Freeman-Browne, D. The aftermath of boxing. Psychol. Med. 3, 270–303 (1973). The first study describing the neuropathology of the condition that is now known as chronic traumatic encephalopathy.
Stein, T. D., Alvarez, V. E. & McKee, A. C. Chronic traumatic encephalopathy: a spectrum of neuropathological changes following repetitive brain trauma in athletes and military personnel. Alzheimers Res. Ther. 6, 4 (2014).
DeKosky, S. T., Blennow, K., Ikonomovic, M. D. & Gandy, S. Acute and chronic traumatic encephalopathies: pathogenesis and biomarkers. Nat. Rev. Neurol. 9, 192–200 (2013).
Johanson, C. E. et al. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res. 5, 10 (2008).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl Med. 4, 147ra111 (2012).
Blennow, K., Hampel, H., Weiner, M. & Zetterberg, H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 6, 131–144 (2010).
Duits, F. H. et al. Performance and complications of lumbar puncture in memory clinics: results of the multicenter lumbar puncture feasibility study. Alzheimers Dement. 12, 154–163 (2016).
Blennow, K. & Nellgård, B. Amyloid beta 1–42 and tau in cerebrospinal fluid after severe traumatic brain injury. Neurology 62, 159 (2004).
Uhlen, M. et al. Proteomics. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Apweiler, R. et al. Approaching clinical proteomics: current state and future fields of application in fluid proteomics. Clin. Chem. Lab. Med. 47, 724–744 (2009).
Bolstad, N., Warren, D. J. & Nustad, K. Heterophilic antibody interference in immunometric assays. Best Pract. Res. Clin. Endocrinol. Metab. 27, 647–661 (2013).
Yoshimura, T. et al. Stability of pro-gastrin-releasing peptide in serum versus plasma. Tumour Biol. 29, 224–230 (2008).
Plog, B. A. et al. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J. Neurosci. 35, 518–526 (2015).
Shi, M. et al. Salivary tau species are potential biomarkers of Alzheimer's disease. J. Alzheimers Dis. 27, 299–305 (2011).
Devic, I. et al. Salivary α-synuclein and DJ-1: potential biomarkers for Parkinson's disease. Brain 134, e178 (2011).
Chen, A. et al. Multiplex analyte assays to characterize different dementias: brain inflammatory cytokines in poststroke and other dementias. Neurobiol. Aging 38, 56–67 (2016).
Rissin, D. M. et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 28, 595–599 (2010).
Todd, J. et al. Ultrasensitive flow-based immunoassays using single-molecule counting. Clin. Chem. 53, 1990–1995 (2007).
Lundberg, M., Eriksson, A., Tran, B., Assarsson, E. & Fredriksson, S. Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res. 39, e102 (2011).
Kricka, L. J. Human anti-animal antibody interferences in immunological assays. Clin. Chem. 45, 942–956 (1999).
Strathmann, F. G. & Hoofnagle, A. N. Current and future applications of mass spectrometry to the clinical laboratory. Am. J. Clin. Pathol. 136, 609–616 (2011).
Sabbagh, B., Mindt, S., Neumaier, M. & Findeisen, P. Clinical applications of MS-based protein quantification. Proteom. Clin. Appl. 10, 323–345 (2016).
Algattas, H. & Huang, J. H. Traumatic brain injury pathophysiology and treatments: early, intermediate, and late phases post-injury. Int. J. Mol. Sci. 15, 309–341 (2014).
Schmechel, D., Marangos, P. J. & Brightman, M. Neurone-specific enolase is a molecular marker for peripheral and central neuroendocrine cells. Nature 276, 834–836 (1978).
Olsson, B., Zetterberg, H., Hampel, H. & Blennow, K. Biomarker-based dissection of neurodegenerative diseases. Prog. Neurobiol. 95, 520–534 (2011).
Dash, P. K., Zhao, J., Hergenroeder, G. & Moore, A. N. Biomarkers for the diagnosis, prognosis, and evaluation of treatment efficacy for traumatic brain injury. Neurotherapeutics 7, 100–114 (2010).
Ramont, L. et al. Effects of hemolysis and storage condition on neuron-specific enolase (NSE) in cerebrospinal fluid and serum: implications in clinical practice. Clin. Chem. Lab. Med. 43, 1215–1217 (2005).
Bohmer, A. E. et al. Neuron-specific enolase, S100B, and glial fibrillary acidic protein levels as outcome predictors in patients with severe traumatic brain injury. Neurosurgery 68, 1624–1630; discussion 1630–1621 (2011).
Chiaretti, A. et al. NGF, DCX, and NSE upregulation correlates with severity and outcome of head trauma in children. Neurology 72, 609–616 (2009).
Varma, S. et al. F2-isoprostane and neuron-specific enolase in cerebrospinal fluid after severe traumatic brain injury in infants and children. J. Neurotrauma 20, 781–786 (2003).
Berger, R. P. et al. Neuron-specific enolase and S100B in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatrics 109, E31 (2002).
Ross, S. A., Cunningham, R. T., Johnston, C. F. & Rowlands, B. J. Neuron-specific enolase as an aid to outcome prediction in head injury. Br. J. Neurosurg. 10, 471–476 (1996).
Pelsers, M. M. et al. Brain- and heart-type fatty acid-binding proteins in the brain: tissue distribution and clinical utility. Clin. Chem. 50, 1568–1575 (2004).
Skogseid, I. M., Nordby, H. K., Urdal, P., Paus, E. & Lilleaas, F. Increased serum creatine kinase BB and neuron specific enolase following head injury indicates brain damage. Acta Neurochir. (Wien) 115, 106–111 (1992).
de Kruijk, J. R., Leffers, P., Menheere, P. P., Meerhoff, S. & Twijnstra, A. S-100B and neuron-specific enolase in serum of mild traumatic brain injury patients. A comparison with health controls. Acta Neurol. Scand. 103, 175–179 (2001).
Wolf, H. et al. Predictive value of neuromarkers supported by a set of clinical criteria in patients with mild traumatic brain injury: S100B protein and neuron-specific enolase on trial: clinical article. J. Neurosurg. 118, 1298–1303 (2013).
Shahim, P. et al. Blood biomarkers for brain injury in concussed professional ice hockey players. JAMA Neurol. 71, 684–692 (2014). A pilot study demonstrating the potential of plasma tau as a biomarker for axonal injury in sports-related concussion.
Chen, F., Sugiura, Y., Myers, K. G., Liu, Y. & Lin, W. Ubiquitin carboxyl-terminal hydrolase L1 is required for maintaining the structure and function of the neuromuscular junction. Proc. Natl Acad. Sci. USA 107, 1636–1641 (2010).
Thompson, R. J., Doran, J. F., Jackson, P., Dhillon, A. P. & Rode, J. PGP. 9.5 — a new marker for vertebrate neurons and neuroendocrine cells. Brain Res. 278, 224–228 (1983).
Takami, Y. et al. Ubiquitin carboxyl-terminal hydrolase L1, a novel deubiquitinating enzyme in the vasculature, attenuates NF-κB activation. Arterioscler. Thromb. Vasc. Biol. 27, 2184–2190 (2007).
Campbell, L. K., Thomas, J. R., Lamps, L. W., Smoller, B. R. & Folpe, A. L. Protein gene product 9.5 (PGP 9.5) is not a specific marker of neural and nerve sheath tumors: an immunohistochemical study of 95 mesenchymal neoplasms. Mod. Pathol. 16, 963–969 (2003).
Brophy, G. M. et al. Biokinetic analysis of ubiquitin C-terminal hydrolase-L1 (UCH-L1) in severe traumatic brain injury patient biofluids. J. Neurotrauma 28, 861–870 (2011).
Kulbe, J. R. & Geddes, J. W. Current status of fluid biomarkers in mild traumatic brain injury. Exp. Neurol. 275, 334–352 (2016).
Papa, L. et al. Serum levels of ubiquitin C-terminal hydrolase distinguish mild traumatic brain injury from trauma controls and are elevated in mild and moderate traumatic brain injury patients with intracranial lesions and neurosurgical intervention. J. Trauma Acute Care Surg. 72, 1335–1344 (2012).
Diaz-Arrastia, R. et al. Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J. Neurotrauma 31, 19–25 (2014).
Kaplan, G. B., Vasterling, J. J. & Vedak, P. C. Brain-derived neurotrophic factor in traumatic brain injury, post-traumatic stress disorder, and their comorbid conditions: role in pathogenesis and treatment. Behav. Pharmacol. 21, 427–437 (2010).
Korley, F. K. et al. Circulating brain-derived neurotrophic factor has diagnostic and prognostic value in traumatic brain injury. J. Neurotrauma 33, 215–225 (2016).
Simon, D., Nascimento, R. I., Filho, E. M., Bencke, J. & Regner, A. Plasma brain-derived neurotrophic factor levels after severe traumatic brain injury. Brain Inj. 30, 23–28 (2016).
Zetterberg, H., Smith, D. H. & Blennow, K. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat. Rev. Neurol. 9, 201–210 (2013).
Morris, M., Maeda, S., Vossel, K. & Mucke, L. The many faces of tau. Neuron 70, 410–426 (2011).
Goedert, M., Spillantini, M. G. & Crowther, R. A. Cloning of a big tau microtubule-associated protein characteristic of the peripheral nervous system. Proc. Natl Acad. Sci. USA 89, 1983–1987 (1992).
Trojanowski, J. Q., Schuck, T., Schmidt, M. L. & Lee, V. M. Distribution of tau proteins in the normal human central and peripheral nervous system. J. Histochem. Cytochem. 37, 209–215 (1989).
Friede, R. L. & Samorajski, T. Axon caliber related to neurofilaments and microtubules in sciatic nerve fibers of rats and mice. Anat. Rec. 167, 379–387 (1970).
Ost, M. et al. Initial CSF total tau correlates with 1-year outcome in patients with traumatic brain injury. Neurology 67, 1600–1604 (2006).
Franz, G. et al. Amyloid beta 1–42 and tau in cerebrospinal fluid after severe traumatic brain injury. Neurology 60, 1457–1461 (2003).
Zemlan, F. P. et al. C-Tau biomarker of neuronal damage in severe brain injured patients: association with elevated intracranial pressure and clinical outcome. Brain Res. 947, 131–139 (2002).
Hesse, C. et al. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci. Lett. 297, 187–190 (2001).
Neselius, S. et al. CSF-biomarkers in Olympic boxing: diagnosis and effects of repetitive head trauma. PLoS ONE 7, e33606 (2012).
Zetterberg, H. et al. Neurochemical aftermath of amateur boxing. Arch. Neurol. 63, 1277–1280 (2006).
Neselius, S., Zetterberg, H., Blennow, K., Marcusson, J. & Brisby, H. Increased CSF levels of phosphorylated neurofilament heavy protein following bout in amateur boxers. PLoS ONE 8, e81249 (2013).
Guzel, A., Karasalihoglu, S., Aylanc, H., Temizoz, O. & Hicdonmez, T. Validity of serum tau protein levels in pediatric patients with minor head trauma. Am. J. Emerg. Med. 28, 399–403 (2010).
Bulut, M. et al. Tau protein as a serum marker of brain damage in mild traumatic brain injury: preliminary results. Adv. Ther. 23, 12–22 (2006).
Kavalci, C. et al. The value of serum tau protein for the diagnosis of intracranial injury in minor head trauma. Am. J. Emerg. Med. 25, 391–395 (2007).
Al Nimer, F. et al. Comparative assessment of the prognostic value of biomarkers in traumatic brain injury reveals an independent role for serum levels of neurofilament light. PLoS ONE 10, e0132177 (2015).
Randall, J. et al. Tau proteins in serum predict neurological outcome after hypoxic brain injury from cardiac arrest: results of a pilot study. Resuscitation 84, 351–356 (2013).
Kuhle, J. et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin. Chem. Lab. Med. http://dx.doi.org/10.1515/cclm-2015-1195 (2016).
Zetterberg, H. et al. Plasma tau levels in Alzheimer's disease. Alzheimers Res. Ther. 5, 9 (2013).
Bogoslovsky, T. et al. Increases of plasma levels of glial fibrillary acidic protein, tau, and amyloid β up to 90 days after traumatic brain injury. J. Neurotrauma http://dx.doi.org/10.1089/neu.2015.4333 (2016).
Olivera, A. et al. Peripheral total tau in military personnel who sustain traumatic brain injuries during deployment. JAMA Neurol. 72, 1109–1116 (2015).
Shahim, P. et al. Serum tau fragments predict return to play in concussed professional ice hockey players. J. Neurotrauma http://dx.doi.org/10.1089/neu.2014.3741 (2016).
Bazarian, J. J., Zemlan, F. P., Mookerjee, S. & Stigbrand, T. Serum S-100B and cleaved-tau are poor predictors of long-term outcome after mild traumatic brain injury. Brain Inj. 20, 759–765 (2006).
Ma, M., Lindsell, C. J., Rosenberry, C. M., Shaw, G. J. & Zemlan, F. P. Serum cleaved tau does not predict postconcussion syndrome after mild traumatic brain injury. Am. J. Emerg. Med. 26, 763–768 (2008).
Gisslen, M. et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine 3, 135–140 (2015).
Oliver, J. et al. Serum neurofilament light in american football athletes over the course of a season. J. Neurotrauma http://dx.doi.org/10.1089/neu.2015.4295 (2016).
Gatson, J. W. et al. Detection of neurofilament-H in serum as a diagnostic tool to predict injury severity in patients who have suffered mild traumatic brain injury. J. Neurosurg. 121, 1232–1238 (2014).
Pike, B. R. et al. Accumulation of non-erythroid αII-spectrin and calpain-cleaved αII-spectrin breakdown products in cerebrospinal fluid after traumatic brain injury in rats. J. Neurochem. 78, 1297–1306 (2001).
Pineda, J. A. et al. Clinical significance of α II-spectrin breakdown products in cerebrospinal fluid after severe traumatic brain injury. J. Neurotrauma 24, 354–366 (2007).
Farkas, O. et al. Spectrin breakdown products in the cerebrospinal fluid in severe head injury — preliminary observations. Acta Neurochir. (Wien) 147, 855–861 (2005).
Mondello, S. et al. alphaII-spectrin breakdown products (SBDPs): diagnosis and outcome in severe traumatic brain injury patients. J. Neurotrauma 27, 1203–1213 (2010).
Czeiter, E. et al. Brain injury biomarkers may improve the predictive power of the IMPACT outcome calculator. J. Neurotrauma 29, 1770–1778 (2012).
Siman, R. et al. A panel of neuron-enriched proteins as markers for traumatic brain injury in humans. J. Neurotrauma 26, 1867–1877 (2009).
Siman, R. et al. Evidence that the blood biomarker SNTF predicts brain imaging changes and persistent cognitive dysfunction in mild TBI patients. Front. Neurol. 4, 190 (2013).
Siman, R. et al. Serum SNTF increases in concussed professional ice hockey players and relates to the severity of post-concussion symptoms. J. Neurotrauma 32, 1294–1300 (2015).
Barbarese, E. et al. Expression and localization of myelin basic protein in oligodendrocytes and transfected fibroblasts. J. Neurochem. 51, 1737–1745 (1988).
Cerri, C. G., Silani, V. & Scarlato, G. Oligoclonal immunoglobulins and immunoreactive myelin basic protein in the cerebrospinal fluid of patients with multiple sclerosis and other neurological diseases. Acta Neurol. (Napoli) 7, 311–314 (1985).
Su, E. et al. Increased CSF concentrations of myelin basic protein after TBI in infants and children: absence of significant effect of therapeutic hypothermia. Neurocrit. Care 17, 401–407 (2012).
Berger, R. P. et al. Serum neuron-specific enolase, S100B, and myelin basic protein concentrations after inflicted and noninflicted traumatic brain injury in children. J. Neurosurg. 103, 61–68 (2005).
Yan, E. B. et al. Post-traumatic hypoxia is associated with prolonged cerebral cytokine production, higher serum biomarker levels, and poor outcome in patients with severe traumatic brain injury. J. Neurotrauma 31, 618–629 (2014).
Unden, J., Ingebrigtsen, T. & Romner, B. Scandinavian guidelines for initial management of minimal, mild and moderate head injuries in adults: an evidence and consensus-based update. BMC Med. 11, 50 (2013).
McMahon, P. J. et al. Measurement of the GFAP-BDP biomarker for the detection of traumatic brain injury compared to computed tomography and magnetic resonance imaging. J. Neurotrauma 32, 527–533 (2015).
Papa, L. et al. GFAP out-performs S100β in detecting traumatic intracranial lesions on computed tomography in trauma patients with mild traumatic brain injury and those with extracranial lesions. J. Neurotrauma 31, 1815–1822 (2014).
Korfias, S. et al. Slight and short-lasting increase of serum S-100B protein in extra-cranial trauma. Brain Inj. 20, 867–872 (2006).
Hay, J. R., Johnson, V. E., Young, A. M., Smith, D. H. & Stewart, W. Blood–brain barrier disruption is an early event that may persist for many years after traumatic brain injury in humans. J. Neuropathol. Exp. Neurol. 74, 1147–1157 (2015).
Rodriguez-Baeza, A., Reina-de la Torre, F., Poca, A., Marti, M. & Garnacho, A. Morphological features in human cortical brain microvessels after head injury: a three-dimensional and immunocytochemical study. Anat. Rec. 273, 583–593 (2003).
Reiber, H. & Peter, J. B. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J. Neurol. Sci. 184, 101–122 (2001).
Brouns, R., Wauters, A., De Surgeloose, D., Marien, P. & De Deyn, P. P. Biochemical markers for blood–brain barrier dysfunction in acute ischemic stroke correlate with evolution and outcome. Eur. Neurol. 65, 23–31 (2011).
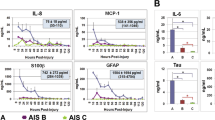
Csuka, E. et al. IL-10 levels in cerebrospinal fluid and serum of patients with severe traumatic brain injury: relationship to IL-6, TNF-α, TGF-β1 and blood–brain barrier function. J. Neuroimmunol. 101, 211–221 (1999).
Kossmann, T. et al. Intrathecal and serum interleukin-6 and the acute-phase response in patients with severe traumatic brain injuries. Shock 4, 311–317 (1995).
Blennow, K. et al. No neurochemical evidence of brain injury after blast overpressure by repeated explosions or firing heavy weapons. Acta Neurol. Scand. 123, 245–251 (2011).
Cummins, P. M. Occludin: one protein, many forms. Mol. Cell. Biol. 32, 242–250 (2012).
Saitou, M. et al. Mammalian occludin in epithelial cells: its expression and subcellular distribution. Eur. J. Cell Biol. 73, 222–231 (1997).
Shan, R. et al. A new panel of blood biomarkers for the diagnosis of mild traumatic brain injury/concussion in adults. J. Neurotrauma 33, 49–57 (2016).
Semple, B. D., Bye, N., Rancan, M., Ziebell, J. M. & Morganti-Kossmann, M. C. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2−/− mice. J. Cereb. Blood Flow Metab. 30, 769–782 (2010).
Kirchhoff, C. et al. Cerebrospinal IL-10 concentration is elevated in non-survivors as compared to survivors after severe traumatic brain injury. Eur. J. Med. Res. 13, 464–468 (2008).
Goodman, J. C., Van, M., Gopinath, S. P. & Robertson, C. S. Pro-inflammatory and pro-apoptotic elements of the neuroinflammatory response are activated in traumatic brain injury. Acta Neurochir. Suppl. 102, 437–439 (2008).
Buttram, S. D. et al. Multiplex assessment of cytokine and chemokine levels in cerebrospinal fluid following severe pediatric traumatic brain injury: effects of moderate hypothermia. J. Neurotrauma 24, 1707–1717 (2007).
Phillips, D. J. et al. Activin a release into cerebrospinal fluid in a subset of patients with severe traumatic brain injury. J. Neurotrauma 23, 1283–1294 (2006).
Maier, B. et al. Delayed elevation of soluble tumor necrosis factor receptors p75 and p55 in cerebrospinal fluid and plasma after traumatic brain injury. Shock 26, 122–127 (2006).
Shiozaki, T. et al. Cerebrospinal fluid concentrations of anti-inflammatory mediators in early-phase severe traumatic brain injury. Shock 23, 406–410 (2005).
Singhal, A. et al. Association between cerebrospinal fluid interleukin-6 concentrations and outcome after severe human traumatic brain injury. J. Neurotrauma 19, 929–937 (2002).
Stahel, P. F. et al. Intrathecal levels of complement-derived soluble membrane attack complex (sC5b-9) correlate with blood–brain barrier dysfunction in patients with traumatic brain injury. J. Neurotrauma 18, 773–781 (2001).
Bell, M. J. et al. Interleukin-6 and interleukin-10 in cerebrospinal fluid after severe traumatic brain injury in children. J. Neurotrauma 14, 451–457 (1997).
Kumar, R. G. et al. Acute CSF interleukin-6 trajectories after TBI: associations with neuroinflammation, polytrauma, and outcome. Brain Behav. Immun. 45, 253–262 (2015).
Csajbok, L. Z., Nylen, K., Ost, M., Sonander, H. & Nellgard, B. In-hospital C-reactive protein predicts outcome after aneurysmal subarachnoid haemorrhage treated by endovascular coiling. Acta Anaesthesiol. Scand. 59, 255–264 (2015).
Berger, R. P., Ta'asan, S., Rand, A., Lokshin, A. & Kochanek, P. Multiplex assessment of serum biomarker concentrations in well-appearing children with inflicted traumatic brain injury. Pediatr. Res. 65, 97–102 (2009).
Lorente, L. New prognostic biomarkers in patients with traumatic brain injury. Arch. Trauma Res. 4, e30165 (2015).
Timofeev, I. et al. Cerebral extracellular chemistry and outcome following traumatic brain injury: a microdialysis study of 223 patients. Brain 134, 484–494 (2011).
Yi, L. et al. Serum metabolic profiling reveals altered metabolic pathways in patients with post-traumatic cognitive impairments. Sci. Rep. 6, 21320 (2016).
Hergenroeder, G. et al. Identification of serum biomarkers in brain-injured adults: potential for predicting elevated intracranial pressure. J. Neurotrauma 25, 79–93 (2008).
Gao, W., Lu, C., Kochanek, P. M. & Berger, R. P. Serum amyloid A is increased in children with abusive head trauma: a gel-based proteomic analysis. Pediatr. Res. 76, 280–286 (2014).
Villapol, S. et al. Hepatic expression of serum amyloid A1 is induced by traumatic brain injury and modulated by telmisartan. Am. J. Pathol. 185, 2641–2652 (2015).
Raad, M. et al. Auto-antibodies in traumatic brain injury and central nervous system trauma. Neuroscience 281, 16–23 (2014).
Marchi, N. et al. Consequences of repeated blood–brain barrier disruption in football players. PLoS ONE 8, e56805 (2013).
Nekludov, M., Mobarrez, F., Gryth, D., Bellander, B. M. & Wallen, H. Formation of microparticles in the injured brain of patients with severe isolated traumatic brain injury. J. Neurotrauma 31, 1927–1933 (2014).
Sheth, S. A., Iavarone, A. T., Liebeskind, D. S., Won, S. J. & Swanson, R. A. Targeted lipid profiling discovers plasma biomarkers of acute brain injury. PLoS ONE 10, e0129735 (2015).
Redell, J. B., Moore, A. N., Ward, N. H., 3rd, Hergenroeder, G. W. & Dash, P. K. Human traumatic brain injury alters plasma microRNA levels. J. Neurotrauma 27, 2147–2156 (2010).
Yang, T. et al. Elevated serum miR-93, miR-191, and miR-499 are noninvasive biomarkers for the presence and progression of traumatic brain injury. J. Neurochem. 137, 122–129 (2016).
Losoi, H. et al. Recovery from mild traumatic brain injury in previously healthy adults. J. Neurotrauma 33, 766–776 (2016).
Babcock, L., Byczkowski, T., Wade, S. L., Ho, M. & Bazarian, J. J. Inability of S100B to predict postconcussion syndrome in children who present to the emergency department with mild traumatic brain injury: a brief report. Pediatr. Emerg. Care 29, 458–461 (2013).
Shahim, P. et al. Neurochemical aftermath of repetitive mild traumatic brain injury. JAMA Neurol. (in the press).
Sundman, M., Doraiswamy, P. M. & Morey, R. A. Neuroimaging assessment of early and late neurobiological sequelae of traumatic brain injury: implications for CTE. Front. Neurosci. 9, 334 (2015).
Corsellis, J. A. Boxing and the brain. BMJ 298, 105–109 (1989).
Small, G. W. et al. PET scanning of brain tau in retired national football league players: preliminary findings. Am. J. Geriatr. Psychiatry 21, 138–144 (2013).
Barrio, J. R. et al. In vivo characterization of chronic traumatic encephalopathy using [F-18]FDDNP PET brain imaging. Proc. Natl Acad. Sci. USA 112, E2039–E2047 (2015).
Kawai, N. et al. Detection of brain amyloid β deposition in patients with neuropsychological impairment after traumatic brain injury: PET evaluation using Pittsburgh Compound-B. Brain Inj. 27, 1026–1031 (2013).
Acknowledgements
Work in the authors' laboratories is supported by the European Research Council, the Swedish Research Council, Swedish State Support for Clinical Research, the Torsten Söderberg Foundation, the Knut and Alice Wallenberg Foundation, VINNOVA and the Wolfson Foundation.
Author information
Authors and Affiliations
Contributions
Both authors contributed to researching literature for the article, and provided substantial contributions to discussion of the content, and to writing, reviewing and editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
H.Z. and K.B. are listed as co-inventors on a US patent application for plasma tau as a brain injury marker, and are co-founders of Brain Biomarker Solutions in Gothenburg AB, a GU Venture-based platform company at the University of Gothenburg. K.B. has served on advisory boards for Eli Lilly, Kyowa Kirin Pharma, Pfizer and Roche.
Related links
PowerPoint slides
Glossary
- Glymphatic system
-
A recently discovered 'waste clearance' pathway from the vertebrate CNS into the bloodstream. Clearance of compounds that accumulate after mild traumatic brain injury through this pathway could influence blood biomarker concentrations.
- Wallerian degeneration
-
When a nerve fibre is injured, the part of the axon separated from the neuron's cell body degenerates distal to the injury — this phenomenon is termed Wallerian degeneration.
- CSF:serum albumin ratio
-
Albumin is produced by the liver, so it has to cross the blood–brain barrier to reach the cerebrospinal fluid (CSF). In healthy humans, the ratio of albumin in CSF versus blood is very small, but if the blood–brain barrier is compromised, the CSF:serum albumin ratio increases.
Rights and permissions
About this article
Cite this article
Zetterberg, H., Blennow, K. Fluid biomarkers for mild traumatic brain injury and related conditions. Nat Rev Neurol 12, 563–574 (2016). https://doi.org/10.1038/nrneurol.2016.127
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2016.127
This article is cited by
-
Enlarged Perivascular Space and Index for Diffusivity Along the Perivascular Space as Emerging Neuroimaging Biomarkers of Neurological Diseases
Cellular and Molecular Neurobiology (2024)
-
Blood biomarkers and neurodegeneration in individuals exposed to repetitive head impacts
Alzheimer's Research & Therapy (2023)
-
Serum glial fibrillary acidic protein and neurofilament light chain as biomarkers of retinal neurodysfunction in early diabetic retinopathy: results of the EUROCONDOR study
Acta Diabetologica (2023)
-
Volumetric MRI Findings in Mild Traumatic Brain Injury (mTBI) and Neuropsychological Outcome
Neuropsychology Review (2023)
-
Variability in the indication of brain CT scan after mild traumatic brain injury. A transnational survey
European Journal of Trauma and Emergency Surgery (2023)