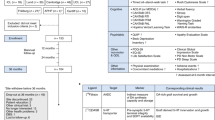
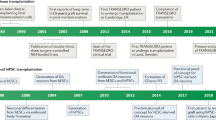
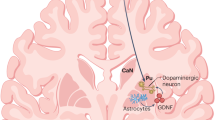
Key Points
-
Dopaminergic drugs were established as an effective treatment for Parkinson disease (PD) in the 1960s, and are still the mainstay of therapy for this condition
-
Experiments that heralded the modern era of neural grafting for PD began in the 1970s in Sweden
-
Despite limited preclinical data, adrenal medullary transplantation was adopted by many groups during the 1980s, with largely disappointing results
-
Human fetal ventral mesencephalic (fVM) allografts have been shown to survive and function for over 20 years in some patients
-
The protocol for neural transplantation in patients with PD remains to be optimized
-
Human fVM grafts are currently being revisited, and stem cell-based dopamine replacement therapies are close to clinical trials
Abstract
Parkinson disease (PD) is characterized by loss of the A9 nigral neurons that provide dopaminergic innervation to the striatum. This discovery led to the successful instigation of dopaminergic drug treatments in the 1960s, although these drugs were soon recognized to lose some of their efficacy and generate their own adverse effects over time. Despite the fact that PD is now known to have extensive non-nigral pathology with a wide range of clinical features, dopaminergic drug therapies are still the mainstay of therapy, and work well for many years. Given the success of pharmacological dopamine replacement, pursuit of cell-based dopamine replacement strategies seemed to be the next logical step, and studies were initiated over 30 years ago to explore the possibility of dopaminergic cell transplantation. In this Review, we outline the history of this therapeutic approach to PD and highlight the lessons that we have learned en route. We discuss how the best clinical outcomes have been obtained with fetal ventral mesencephalic allografts, while acknowledging inconsistencies in the results owing to problems in trial design, patient selection, tissue preparation, and immunotherapy used post-grafting. We conclude by discussing the challenges of bringing the new generation of stem cell-derived dopamine cells to the clinic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Spillantini, M. G. et al. α-Synuclein in Lewy bodies. Nature 388, 839–840 (1997).
Damier, P., Hirsch, E. C., Agid, Y. & Graybiel, A. M. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain 122, 1437–1448 (1999).
Braak, H. et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging 24, 197–211 (2003).
Jenner, P. Dopamine agonists, receptor selectivity and dyskinesia induction in Parkinson's disease. Curr. Opin. Neurol. 16 (Suppl. 1), S3–S7 (2003).
Huot, P., Johnston, T. H., Koprich, J. B., Fox, S. H. & Brotchie, J. M. The pharmacology of L-DOPA-induced dyskinesia in Parkinson's disease. Pharmacol. Rev. 65, 171–222 (2013).
Thompson, W. G. Successful brain grafting. N. Y. Med. J. 51, 701–702 (1890).
Olson, L. & Seiger, A. Brain tissue transplanted to the anterior chamber of the eye: 2. Fluorescence histochemistry of immature catecholamine- and 5-hydroxytryptamine neurons innervating the rat vas deferens. Cell Tissue Res. 158, 141–150 (1975).
Olson, L. & Seiger, A. Development and growth of immature monoamine neurons in rat and man in situ and following intraocular transplantation in the rat. Brain Res. 62, 353–360 (1973).
Olson, L. & Seiger, A. Brain tissue transplanted to the anterior chamber of the eye. 1. Fluorescence histochemistry of immature catecholamine and 5-hydroxytryptamine neurons reinnervating the rat iris. Z. Zellforsch. Mikrosk. Anat. 135, 175–194 (1972).
Barker, R. & Dunnett, S. The biology and behaviour of intracerebral adrenal transplants in animals and man. Rev. Neurosci. 4, 113–146 (1993).
Ungerstedt, U., Ljungberg, T. & Steg, G. Behavioral, physiological, and neurochemical changes after 6-hydroxydopamine-induced degeneration of the nigro-striatal dopamine neurons. Adv. Neurol. 5, 421–426 (1974).
Ungerstedt, U. & Arbuthnott, G. W. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 24, 485–493 (1970).
Ungerstedt, U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur. J. Pharmacol. 5, 107–110 (1968).
Hudson, J. L. et al. Correlation of apomorphine- and amphetamine-induced turning with nigrostriatal dopamine content in unilateral 6-hydroxydopamine lesioned rats. Brain Res. 626, 167–174 (1993).
Freed, W. J. et al. Transplanted adrenal chromaffin cells in rat brain reduce lesion-induced rotational behaviour. Nature 292, 351–352 (1981).
Perlow, M. J. et al. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science 204, 643–647 (1979).
Hoffer, B., Freed, W., Olson, L. & Wyatt, R. J. Transplantation of dopamine-containing tissues to the central nervous system. Clin. Neurosurg. 31, 404–416 (1983).
Freed, W. J. et al. Restoration of dopaminergic function by grafting of fetal rat substantia nigra to the caudate nucleus: long-term behavioral, biochemical, and histochemical studies. Ann. Neurol. 8, 510–519 (1980).
Björklund, A., Stenevi, U., Dunnett, S. B. & Iversen, S. D. Functional reactivation of the deafferented neostriatum by nigral transplants. Nature 289, 497–499 (1981).
Björklund, A., Dunnett, S. B., Stenevi, U., Lewis, M. E. & Iversen, S. D. Reinnervation of the denervated striatum by substantia nigra transplants: functional consequences as revealed by pharmacological and sensorimotor testing. Brain Res. 199, 307–333 (1980).
Björklund, A. & Stenevi, U. Reconstruction of the nigrostriatal dopamine pathway by intracerebral nigral transplants. Brain Res. 177, 555–560 (1979).
Björklund, A., Stenevi, U., Schmidt, R. H., Dunnett, S. B. & Gage, F. H. Intracerebral grafting of neuronal cell suspensions. II. Survival and growth of nigral cell suspensions implanted in different brain sites. Acta Physiol. Scand. Suppl. 522, 9–18 (1983).
Brundin, P., Barker, R. A. & Parmar, M. Neural grafting in Parkinson's disease: problems and possibilities. Prog. Brain Res. 184, 265–294 (2010).
Barker, R. A. What have open label studies of cell based therapies for Parkinson's disease told us, if anything? Basal Ganglia 4, 85–87 (2014).
Backlund, E. O. et al. Transplantation of adrenal medullary tissue to striatum in parkinsonism. First clinical trials. J. Neurosurg. 62, 169–173 (1985).
Freed, W. J., Poltorak, M. & Becker, J. B. Intracerebral adrenal medulla grafts: a review. Exp. Neurol. 110, 139–166 (1990).
Madrazo, I. et al. Open microsurgical autograft of adrenal medulla to the right caudate nucleus in two patients with intractable Parkinson's disease. N. Engl. J. Med. 316, 831–834 (1987).
Moore, R. Y. Parkinson's disease—a new therapy? N. Engl. J. Med. 316, 872–873 (1987).
Allen, G. S., Burns, R. S., Tulipan, N. B. & Parker, R. A. Adrenal medullary transplantation to the caudate nucleus in Parkinson's disease. Initial clinical results in 18 patients. Arch. Neurol. 46, 487–491 (1989).
Drucker-Colin, R. et al. Adrenal medullary tissue transplants in the caudate nucleus of Parkinson's patients. Prog. Brain Res. 78, 567–574 (1988).
Goetz, C. G. et al. Multicenter study of autologous adrenal medullary transplantation to the corpus striatum in patients with advanced Parkinson's disease. N. Engl. J. Med. 320, 337–341 (1989).
Goetz, C. G. et al. Adrenal medullary transplant to the striatum of patients with advanced Parkinson's disease: 1-year motor and psychomotor data. Neurology 40, 273–276 (1990).
Jankovic, J. et al. Clinical, biochemical, and neuropathologic findings following transplantation of adrenal medulla to the caudate nucleus for treatment of Parkinson's disease. Neurology 39, 1227–1234 (1989).
Jiao, S. S. et al. Study of adrenal medullary tissue transplantation to striatum in parkinsonism. Prog. Brain Res. 78, 575–580 (1988).
Jiao, S. S. et al. Adrenal medullary autografts in patients with Parkinson's disease. N. Engl. J. Med. 321, 324–327 (1989).
Kelly, P. J. et al. Adrenal medullary autograft transplantation into the striatum of patients with Parkinson's disease. Mayo Clin. Proc. 64, 282–290 (1989).
Lindvall, O. et al. Transplantation in Parkinson's disease: two cases of adrenal medullary grafts to the putamen. Ann. Neurol. 22, 457–468 (1987).
Ostrosky-Solis, F. et al. Neuropsychological effects of brain autograft of adrenal medullary tissue for the treatment of Parkinson's disease. Neurology 38, 1442–1450 (1988).
Goetz, C. G. et al. United Parkinson Foundation Neurotransplantation Registry on adrenal medullary transplants: presurgical, and 1- and 2-year follow-up. Neurology 41, 1719–1722 (1991).
Hurtig, H., Joyce, J., Sladek, J. R. J. & Trojanowski, J. Q. Postmortem analysis of adrenal-medulla-to-caudate autograft in a patient with Parkinson's disease. Ann. Neurol. 25, 607–614 (1989).
Kompoliti, K., Chu, Y., Shannon, K. M. & Kordower, J. H. Neuropathological study 16 years after autologous adrenal medullary transplantation in a Parkinson's disease patient. Mov. Disord. 22, 1630–1633 (2007).
Kordower, J. H., Cochran, E., Penn, R. D. & Goetz, C. G. Putative chromaffin cell survival and enhanced host-derived TH-fiber innervation following a functional adrenal medulla autograft for Parkinson's disease. Ann. Neurol. 29, 405–412 (1991).
Waters, C., Itabashi, H. H., Apuzzo, M. L. & Weiner, L. P. Adrenal to caudate transplantation—postmortem study. Mov. Disord. 5, 248–250 (1990).
Lindvall, O. et al. Human fetal dopamine neurons grafted into the striatum in two patients with severe Parkinson's disease. A detailed account of methodology and a 6-month follow-up. Arch. Neurol. 46, 615–631 (1989).
Lindvall, O. et al. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson's disease. Science 247, 574–577 (1990).
Brundin, P. et al. Bilateral caudate and putamen grafts of embryonic mesencephalic tissue treated with lazaroids in Parkinson's disease. Brain 123, 1380–1390 (2000).
Lindvall, O. et al. Evidence for long-term survival and function of dopaminergic grafts in progressive Parkinson's disease. Ann. Neurol. 35, 172–180 (1994).
Wenning, G. K. et al. Short- and long-term survival and function of unilateral intrastriatal dopaminergic grafts in Parkinson's disease. Ann. Neurol. 42, 95–107 (1997).
Piccini, P. et al. Dopamine release from nigral transplants visualized in vivo in a Parkinson's patient. Nat. Neurosci. 2, 1137–1140 (1999).
Piccini, P. et al. Delayed recovery of movement-related cortical function in Parkinson's disease after striatal dopaminergic grafts. Ann. Neurol. 48, 689–695 (2000).
Kefalopoulou, Z. et al. Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol. 71, 83–87 (2014).
Freed, C. R. et al. Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinson's disease. N. Engl. J. Med. 327, 1549–1555 (1992).
Freeman, T. B. et al. Bilateral fetal nigral transplantation into the postcommissural putamen in Parkinson's disease. Ann. Neurol. 38, 379–388 (1995).
Redmond, D. E. et al. Cellular replacement of dopamine deficit in Parkinson's disease using human fetal mesencephalic tissue: preliminary results in four patients. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 71, 325–359 (1993).
Widner, H. et al. Bilateral fetal mesencephalic grafting in two patients with parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). N. Engl. J. Med. 327, 1556–1563 (1992).
Mendez, I. et al. Enhancement of survival of stored dopaminergic cells and promotion of graft survival by exposure of human fetal nigral tissue to glial cell line-derived neurotrophic factor in patients with Parkinson's disease. Report of two cases and technical considerations. J. Neurosurg. 92, 863–869 (2000).
Mendez, I. et al. Simultaneous intrastriatal and intranigral fetal dopaminergic grafts in patients with Parkinson disease: a pilot study. Report of three cases. J. Neurosurg. 96, 589–596 (2002).
Widner, H. NIH neural transplantation funding. Science 263, 737 (1994).
Kumar, R. et al. Double-blind evaluation of subthalamic nucleus deep brain stimulation in advanced Parkinson's disease. Neurology 51, 850–855 (1998).
Freed, C. R. et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N. Engl. J. Med. 344, 710–719 (2001).
Olanow, C. W. et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann. Neurol. 54, 403–414 (2003).
Cho, C., Alterman, R., Miravite, J., Shils, J. & Tagliati, M. Subthalamic DBS for the treatment of “runaway” dyskinesias after embryonic or fetal tissue transplant. Mov. Disord. 20, 1237 (2005).
Graff-Radford, J. et al. Deep brain stimulation of the internal segment of the globus pallidus in delayed runaway dyskinesia. Arch. Neurol. 63, 1181–1184 (2006).
Herzog, J. et al. Deep brain stimulation in Parkinson's disease following fetal nigral transplantation. Mov. Disord. 23, 1293–1296 (2008).
Hagell, P. et al. Dyskinesias following neural transplantation in Parkinson's disease. Nat. Neurosci. 5, 627–628 (2002).
Ma, Y. et al. Dyskinesia after fetal cell transplantation for parkinsonism: a PET study. Ann. Neurol. 52, 628–634 (2002).
Barker, R. A. & Kuan, W. L. Graft-induced dyskinesias in Parkinson's disease: what is it all about? Cell Stem Cell 7, 148–149 (2010).
Politis, M. et al. Serotonergic neurons mediate dyskinesia side effects in Parkinson's patients with neural transplants. Sci. Transl. Med. 2, 38ra46 (2010).
Politis, M. et al. Graft-induced dyskinesias in Parkinson's disease: high striatal serotonin/dopamine transporter ratio. Mov. Disord. 26, 1997–2003 (2011).
Lane, E. L., Winkler, C., Brundin, P. & Cenci, M. A. The impact of graft size on the development of dyskinesia following intrastriatal grafting of embryonic dopamine neurons in the rat. Neurobiol. Dis. 22, 334–345 (2006).
Winkler, C., Georgievska, B., Carlsson, T., Lacar, B. & Kirik, D. Continuous exposure to glial cell line-derived neurotrophic factor to mature dopaminergic transplants impairs the graft's ability to improve spontaneous motor behavior in parkinsonian rats. Neuroscience 141, 521–531 (2006).
Mendez, I. et al. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson's disease. Brain 128, 1498–1510 (2005).
Krack, P., Poepping, M., Weinert, D., Schrader, B. & Deuschl, G. Thalamic, pallidal, or subthalamic surgery for Parkinson's disease? J. Neurol. 247 (Suppl. 2), II122–II134 (2000).
Ma, Y. et al. Dopamine cell implantation in Parkinson's disease: long-term clinical and 18F-FDOPA PET outcomes. J. Nucl. Med. 51, 7–15 (2010).
Barker, R. A., Barrett, J., Mason, S. L. & Björklund, A. Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson's disease. Lancet Neurol. 12, 84–91 (2013).
Piccini, P. et al. Factors affecting the clinical outcome after neural transplantation in Parkinson's disease. Brain 128, 2977–2986 (2005).
Kordower, J. H. et al. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson's disease. N. Engl. J. Med. 332, 1118–1124 (1995).
Kordower, J. H. et al. Functional fetal nigral grafts in a patient with Parkinson's disease: chemoanatomic, ultrastructural, and metabolic studies. J. Comp. Neurol. 370, 203–230 (1996).
Kordower, J. H. et al. Fetal nigral grafts survive and mediate clinical benefit in a patient with Parkinson's disease. Mov. Disord. 13, 383–393 (1998).
TRANSEURO [online], (2014).
Barker, R. A., Kendall, A. L. & Widner, H. Neural tissue xenotransplantation: what is needed prior to clinical trials in Parkinson's disease? Neural Tissue Xenografting Project. Cell Transplant. 9, 235–246 (2000).
Galpern, W. R., Burns, L. H., Deacon, T. W., Dinsmore, J. & Isacson, O. Xenotransplantation of porcine fetal ventral mesencephalon in a rat model of Parkinson's disease: functional recovery and graft morphology. Exp. Neurol. 140, 1–13 (1996).
Schumacher, J. M. et al. Transplantation of embryonic porcine mesencephalic tissue in patients with PD. Neurology 54, 1042–1050 (2000).
Arjona, V. et al. Autotransplantation of human carotid body cell aggregates for treatment of Parkinson's disease. Neurosurgery 53, 321–328 (2003).
Minguez-Castellanos, A. et al. Carotid body autotransplantation in Parkinson disease: a clinical and positron emission tomography study. J. Neurol. Neurosurg. Psychiatry 78, 825–831 (2007).
Bakay, R. A. et al. Implantation of Spheramine in advanced Parkinson's disease (PD). Front. Biosci. 9, 592–602 (2004).
Stover, N. P. et al. Intrastriatal implantation of human retinal pigment epithelial cells attached to microcarriers in advanced Parkinson disease. Arch. Neurol. 62, 1833–1837 (2005).
Stover, N. P. & Watts, R. L. Spheramine for treatment of Parkinson's disease. Neurotherapeutics 5, 252–259 (2008).
Watts, R. L. et al. Stereotaxic intrastriatal implantation of human retinal pigment epithelial (hRPE) cells attached to gelatin microcarriers: a potential new cell therapy for Parkinson's disease. J. Neural Transm. Suppl. 65, 215–227 (2003).
Gross, R. E. et al. Intrastriatal transplantation of microcarrier-bound human retinal pigment epithelial cells versus sham surgery in patients with advanced Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol. 10, 509–519 (2011).
Ribeiro, D. et al. Efficient expansion and dopaminergic differentiation of human fetal ventral midbrain neural stem cells by midbrain morphogens. Neurobiol. Dis. 49, 118–127 (2013).
Barker, R. A. & de Beaufort, I. Scientific and ethical issues related to stem cell research and interventions in neurodegenerative disorders of the brain. Prog. Neurobiol. 110, 63–73 (2013).
Barker, R. A. Developing stem cell therapies for Parkinson's disease: waiting until the time is right. Cell Stem Cell 15, 539–542 (2014).
Thomson, J. A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998).
Reubinoff, B. E., Pera, M. F., Fong, C. Y., Trounson, A. & Bongso, A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat. Biotechnol. 18, 399–404 (2000).
Itskovitz-Eldor, J. et al. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol. Med. 6, 88–95 (2000).
Reubinoff, B. E. et al. Neural progenitors from human embryonic stem cells. Nat. Biotechnol. 19, 1134–1140 (2001).
Zhang, S. C., Wernig, M., Duncan, I. D., Brustle, O. & Thomson, J. A. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 19, 1129–1133 (2001).
Kawasaki, H. et al. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron 28, 31–40 (2000).
Kim, J. H. et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature 418, 50–56 (2002).
Brederlau, A. et al. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson's disease: effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells 24, 1433–1440 (2006).
Park, C. H. et al. In vitro and in vivo analyses of human embryonic stem cell-derived dopamine neurons. J. Neurochem. 92, 1265–1276 (2005).
Perrier, A. L. et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc. Natl Acad. Sci. USA 101, 12543–12548 (2004).
Sonntag, K. C. et al. Enhanced yield of neuroepithelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the bone morphogenic protein antagonist noggin. Stem Cells 25, 411–418 (2007).
Zeng, X. et al. Dopaminergic differentiation of human embryonic stem cells. Stem Cells 22, 925–940 (2004).
Roy, N. S. et al. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat. Med. 12, 1259–1268 (2006).
Cooper, O. et al. Differentiation of human ES and Parkinson's disease iPS cells into ventral midbrain dopaminergic neurons requires a high activity form of SHH, FGF8a and specific regionalization by retinoic acid. Mol. Cell. Neurosci. 45, 258–266 (2010).
Yan, Y. et al. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells 23, 781–790 (2005).
Yang, D., Zhang, Z. J., Oldenburg, M., Ayala, M. & Zhang, S. C. Human embryonic stem cell-derived dopaminergic neurons reverse functional deficit in parkinsonian rats. Stem Cells 26, 55–63 (2008).
Takahashi, K., Okita, K., Nakagawa, M. & Yamanaka, S. Induction of pluripotent stem cells from fibroblast cultures. Nat. Protoc. 2, 3081–3089 (2007).
Soldner, F. et al. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell 136, 964–977 (2009).
Hargus, G. et al. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in parkinsonian rats. Proc. Natl Acad. Sci. USA 107, 15921–15926 (2010).
Kikuchi, T. et al. Survival of human induced pluripotent stem cell-derived midbrain dopaminergic neurons in the brain of a primate model of Parkinson's disease. J. Parkinsons Dis. 1, 395–412 (2011).
Bonilla, S. et al. Identification of midbrain floor plate radial glia-like cells as dopaminergic progenitors. Glia 56, 809–820 (2008).
Ono, Y. et al. Differences in neurogenic potential in floor plate cells along an anteroposterior location: midbrain dopaminergic neurons originate from mesencephalic floor plate cells. Development 134, 3213–3225 (2007).
Placzek, M. & Briscoe, J. The floor plate: multiple cells, multiple signals. Nat. Rev. Neurosci. 6, 230–240 (2005).
Arenas, E., Denham, M. & Villaescusa, J. C. How to make a midbrain dopaminergic neuron. Development 142, 1918–1936 (2015).
Chambers, S. M. et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 27, 275–280 (2009).
Tabar, V. & Studer, L. Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nat. Rev. Genet. 15, 82–92 (2014).
Fasano, C. A., Chambers, S. M., Lee, G., Tomishima, M. J. & Studer, L. Efficient derivation of functional floor plate tissue from human embryonic stem cells. Cell Stem Cell 6, 336–347 (2010).
Kirkeby, A. et al. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 1, 703–714 (2012).
Kriks, S. et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature 480, 547–551 (2011).
Grealish, S. et al. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson's disease. Cell Stem Cell 15, 653–665 (2014).
Grealish, S. et al. Monosynaptic tracing using modified rabies virus reveals early and extensive circuit integration of human embryonic stem cell-derived neurons. Stem Cell Rep. http://dx.doi.org/10.1016/j.stemcr.2015.04.011.
Steinbeck, J. A. et al. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson's disease model. Nat. Biotechnol. 33, 204–209 (2015).
Rath, A. et al. Survival and functional restoration of human fetal ventral mesencephalon following transplantation in a rat model of Parkinson's disease. Cell Transplant. 22, 1281–1293 (2013).
Alper, J. Geron gets green light for human trial of ES cell-derived product. Nat. Biotechnol. 27, 213–214 (2009).
Kanemura, H. et al. Tumorigenicity studies of induced pluripotent stem cell (iPSC)-Derived retinal pigment epithelium (RPE) for the treatment of age-related macular degeneration. PLoS ONE 9, e85336 (2014).
Kordower, J. H., Chu, Y., Hauser, R. A., Freeman, T. B. & Olanow, C. W. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat. Med. 14, 504–506 (2008).
Li, J. Y. et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat. Med. 14, 501–503 (2008).
Chu, Y. & Kordower, J. H. Lewy body pathology in fetal grafts. Ann. N. Y. Acad. Sci. 1184, 55–67 (2010).
Li, J. Y. et al. Characterization of Lewy body pathology in 12- and 16-year-old intrastriatal mesencephalic grafts surviving in a patient with Parkinson's disease. Mov. Disord. 25, 1091–1096 (2010).
Guo, J. L. & Lee, V. M. Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat. Med. 20, 130–138 (2014).
Hallett, P. J. et al. Long-term health of dopaminergic neuron transplants in Parkinson's disease patients. Cell Rep. 7, 1755–1761 (2014).
Abbott, A. Fetal-cell revival for Parkinson's. Nature 510, 195–196 (2014).
GForce-PD [online], (2015).
Hirsch, E. C., Duyckaerts, C., Javoy-Agid, F., Hauw, J. J. & Agid, Y. Does adrenal graft enhance recovery of dopaminergic neurons in Parkinson's disease? Ann. Neurol. 27, 676–682 (1990).
Peterson, D. I., Price, M. L. & Small, C. S. Autopsy findings in a patient who had an adrenal-to-brain transplant for Parkinson's disease. Neurology 39, 235–238 (1989).
Olanow, C. W. et al. Autologous transplantation of adrenal medulla in Parkinson's disease. 18-month results. Arch. Neurol. 47, 1286–1289 (1990).
Hallett, P. J. et al. Successful function of autologous iPSC-derived dopamine neurons following transplantation in a non-human primate model of Parkinson's disease. Cell Stem Cell 16, 269–274 (2015).
Delcroix, G. J. et al. The therapeutic potential of human multipotent mesenchymal stromal cells combined with pharmacologically active microcarriers transplanted in hemi-parkinsonian rats. Biomaterials 32, 1560–1573 (2011).
Offen, D. et al. Intrastriatal transplantation of mouse bone marrow-derived stem cells improves motor behavior in a mouse model of Parkinson's disease. J. Neural Transm. Suppl. 133–143 (2007).
Sanchez-Pernaute, R., Studer, L., Bankiewicz, K. S., Major, E. O. & McKay, R. D. In vitro generation and transplantation of precursor-derived human dopamine neurons. J. Neurosci. Res. 65, 284–288 (2001).
Caiazzo, M. et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 476, 224–227 (2011).
Pfisterer, U. et al. Direct conversion of human fibroblasts to dopaminergic neurons. Proc. Natl Acad. Sci. USA 108, 10343–10348 (2011).
Acknowledgements
The authors' own work is supported by grants from Neurostemcellrepair (grant no. 602278) and the Swedish Research Council (grants K2012-99X-22324-01-5 and K2014-61X-20391-08-4) and TRANSEURO, and by the National institute for Health Research (NIHR)-funded Biomedical Research Centre in Cambridge, UK. M.P. is funded from the European Research Council ERC Grant Agreement no. 309712. We would also like to thank Hakan Widner, Olle Lindvall and Anders Björklund for their advice and input, especially relating to information on the patients grafted in Lund.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article and reviewed and/or edited the manuscript before submission. R.A.B. and M.P. discussed the content and wrote the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Barker, R., Drouin-Ouellet, J. & Parmar, M. Cell-based therapies for Parkinson disease—past insights and future potential. Nat Rev Neurol 11, 492–503 (2015). https://doi.org/10.1038/nrneurol.2015.123
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2015.123
This article is cited by
-
Co-transplantation of autologous Treg cells in a cell therapy for Parkinson’s disease
Nature (2023)
-
Optimizing maturity and dose of iPSC-derived dopamine progenitor cell therapy for Parkinson’s disease
npj Regenerative Medicine (2022)
-
Spotting-based differentiation of functional dopaminergic progenitors from human pluripotent stem cells
Nature Protocols (2022)
-
piRNAs Interact with Cold-Shock Domain-Containing RNA Binding Proteins and Regulate Neuronal Gene Expression During Differentiation
Molecular Neurobiology (2022)
-
Novel targeted therapies for Parkinson’s disease
Molecular Medicine (2021)