Key Points
-
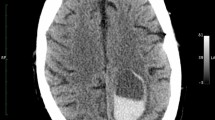
Secondary injury following intracerebral haemorrhage (ICH) is caused by perihaemorrhagic inflammation, toxic products of blood breakdown, and perihaematomal oedema (PHO)
-
Secondary injury contributes to neurological deterioration over an extended period of hours to days and, therefore, offers a long therapeutic window
-
PHO forms in three stages in accordance with Starling's principle: stage 1 is characterized by ionic oedema, and stages 2 and 3 by progressive vasogenic oedema
-
PHO is a pathophysiological marker of secondary injury that could provide a useful surrogate end point for testing novel neuroprotective agents
-
PHO might also be clinically relevant, as it augments the mass effect of haemorrhage; further studies could identify subgroups of patients who would benefit from therapies that ameliorate PHO
-
Combination treatment regimens that target different stages of PHO formation might be most effective to reduce swelling
Abstract
Perihaematomal oedema (PHO) is an important pathophysiological marker of secondary injury in intracerebral haemorrhage (ICH). In this Review, we describe a novel method to conceptualize PHO formation within the framework of Starling's principle of movement of fluid across a capillary wall. We consider progression of PHO through three stages, characterized by ionic oedema (stage 1) and progressive vasogenic oedema (stages 2 and 3). In this context, possible modifiers of PHO volume and their value in identifying patients who would benefit from therapies that target secondary injury are discussed; the practicalities of using neuroimaging to measure PHO volume are also considered. We examine whether PHO can be used as a predictor of neurological outcome following ICH, and we provide an overview of emerging therapies. Our discussion emphasizes that PHO has clinical relevance both as a therapeutic target, owing to its augmentation of the mass effect of a haemorrhage, and as a surrogate marker for novel interventions that target secondary injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
van Asch, C. J. et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 9, 167–176 (2010).
Keep, R. F., Hua, Y. & Xi, G. H. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 11, 720–731 (2012).
Brouwers, H. B. & Greenberg, S. M. Hematoma expansion following acute intracerebral hemorrhage. Cerebrovasc. Dis. 35, 195–201 (2013).
Li, N. et al. Association of molecular markers with perihematomal edema and clinical outcome in intracerebral hemorrhage. Stroke 44, 658–663 (2013).
Wang, J. & Tsirka, S. E. Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain 128, 1622–1633 (2005).
Li, G. et al. Neuroprotective effects of argatroban and C5a receptor antagonist (PMX53) following intracerebral haemorrhage. Clin. Exp. Immunol. 175, 285–295 (2014).
Appelboom, G. et al. Volume-dependent effect of perihaematomal oedema on outcome for spontaneous intracerebral haemorrhages. J. Neurol. Neurosurg. Psychiatry 84, 488–493 (2013).
Arima, H. et al. Significance of perihematomal edema in acute intracerebral hemorrhage: the INTERACT trial. Neurology 73, 1963–1968 (2009).
Xi, G. H., Keep, R. F. & Hoff, J. T. Pathophysiology of brain edema formation. Neurosurg. Clin. N. Am. 13, 371–383 (2002).
Simard, J. M., Kent, T. A., Chen, M. K., Tarasov, K. V. & Gerzanich, V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 6, 258–268 (2007).
Kahle, K. T. et al. Molecular mechanisms of ischemic cerebral edema: role of electroneutral ion transport. Physiology (Bethesda) 24, 257–265 (2009).
Venkatasubramanian, C. et al. Natural history of perihematomal edema after intracerebral hemorrhage measured by serial magnetic resonance imaging. Stroke 42, 73–80 (2011).
Inaji, M. et al. Chronological changes of perihematomal edema of human intracerebral hematoma. Acta Neurochir. Suppl. 86, 445–448 (2003).
Suga, S., Sato, S., Yunoki, K. & Mihara, B. Sequential change of brain edema by semiquantitative measurement on MRI in patients with hypertensive intracerebral hemorrhage. Acta Neurochir. Suppl. (Wien) 60, 564–567 (1994).
Wagner, K. R. et al. Lobar intracerebral hemorrhage model in pigs—rapid edema development in perihematomal white matter. Stroke 27, 490–497 (1996).
Brunswick, A. S. et al. Serum biomarkers of spontaneous intracerebral hemorrhage induced secondary brain injury. J. Neurol. Sci. 321, 1–10 (2012).
MacAulay, N., Hamann, S. & Zeuthen, T. Water transport in the brain: role of cotransporters. Neuroscience 129, 1031–1044 (2004).
Pohl, P. Combined transport of water and ions through membrane channels. Biol. Chem. 385, 921–926 (2004).
Hoomann, T., Jahnke, N., Horner, A., Keller, S. & Pohl, P. Filter gate closure inhibits ion but not water transport through potassium channels. Proc. Natl Acad. Sci. USA 110, 10842–10847 (2013).
Lippoldt, A. et al. Organization of choroid plexus epithelial and endothelial cell tight junctions and regulation of claudin-1, -2 and -5 expression by protein kinase C. Neuroreport 11, 1427–1431 (2000).
Xu, J. et al. Internalization of aquaporin-4 after collagenase-induced intracerebral hemorrhage. Anat. Rec. http://dx.doi.org/10.1002/ar.23055
Stokum, J. A., Kurland, D. B., Gerzanich, V. & Simard, J. M. Mechanisms of astrocyte-mediated cerebral edema. Neurochem. Res. http://dx.doi.org/10.1007/s11064-014-1374-3.
Simard, J. M., Kahle, K. T. & Gerzanich, V. Molecular mechanisms of microvascular failure in central nervous system injury—synergistic roles of NKCC1 and SUR1/TRPM4. J. Neurosurg. 113, 622–629 (2010).
Dziedzic, T. et al. Intracerebral hemorrhage triggers interleukin-6 and interleukin-10 release in blood. Stroke 33, 2334–2335 (2002).
Qureshi, A. I. et al. Extracellular glutamate and other amino acids in experimental intracerebral hemorrhage: an in vivo microdialysis study. Crit. Care Med. 31, 1482–1489 (2003).
Simard, J. M. et al. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 29, 317–330 (2009).
Tosun, C. et al. Inhibition of the Sur1–Trpm4 channel reduces neuroinflammation and cognitive impairment in subarachnoid hemorrhage. Stroke 44, 3522–3528 (2013).
Simard, J. M., Woo, S. K., Bhatta, S. & Gerzanich, V. Drugs acting on SUR1 to treat CNS ischemia and trauma. Curr. Opin. Pharmacol. 8, 42–49 (2008).
Aronowski, J. & Zhao, X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke 42, 1781–1786 (2011).
Wang, J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog. Neurobiol. 92, 463–477 (2010).
Zhao, X. R. et al. Distinct patterns of intracerebral hemorrhage-induced alterations in NF-κB subunit, iNOS, and COX-2 expression. J. Neurochem. 101, 652–663 (2007).
Sansing, L. H. et al. Toll-like receptor 4 contributes to poor outcome after intracerebral hemorrhage. Ann. Neurol. 70, 646–656 (2011).
Bodmer, D., Vaughan, K. A., Zacharia, B. E., Hickman, Z. L. & Connolly, E. S. The molecular mechanisms that promote edema after intracerebral hemorrhage. Transl. Stroke Res. 3 (Suppl. 1), 52–61 (2012).
Wang, Y. C. et al. Toll-like receptor 2/4 heterodimer mediates inflammatory injury in intracerebral hemorrhage. Ann. Neurol. 75, 876–889 (2014).
Suo, Z., Wu, M., Citron, B. A., Gao, C. & Festoff, B. W. Persistent protease-activated receptor 4 signaling mediates thrombin-induced microglial activation. J. Biol. Chem. 278, 31177–31183 (2003).
Lei, B. et al. Tumor necrosis factor alpha antagonism improves neurological recovery in murine intracerebral hemorrhage. J. Neuroinflammation 10, 103 (2013).
Masada, T. et al. Attenuation of intracerebral hemorrhage and thrombin-induced brain edema by overexpression of interleukin-1 receptor antagonist. J. Neurosurg. 95, 680–686 (2001).
Megyeri, P. et al. Recombinant human tumor necrosis factor alpha constricts pial arterioles and increases blood–brain barrier permeability in newborn piglets. Neurosci. Lett. 148, 137–140 (1992).
Aslam, M., Ahmad, N., Srivastava, R. & Hemmer, B. TNF-alpha induced NFκB signaling and p65 (RelA) overexpression repress Cldn5 promoter in mouse brain endothelial cells. Cytokine 57, 269–275 (2012).
Bartha, K., Dömötör, E., Lanza, F., Adam-Vizi, V. & Machovich, R. Identification of thrombin receptors in rat brain capillary endothelial cells. J. Cereb. Blood Flow Metab. 20, 175–182 (2000).
Zheng, G. Q. et al. Long-time course of protease-activated receptor-1 expression after intracerebral hemorrhage in rats. Neurosci. Lett. 459, 62–65 (2009).
Zhou, Z. H., Qu, F. & Zhang, C. D. Systemic administration of argatroban inhibits protease-activated receptor-1 expression in perihematomal tissue in rats with intracerebral hemorrhage. Brain Res. Bull. 86, 235–238 (2011).
Satpathy, M., Gallagher, P., Lizotte-Waniewski, M. & Srinivas, S. P. Thrombin-induced phosphorylation of the regulatory light chain of myosin II in cultured bovine corneal endothelial cells. Exp. Eye Res. 79, 477–486 (2004).
Bijli, K. M. et al. c-Src interacts with and phosphorylates RelA/p65 to promote thrombin-induced ICAM-1 expression in endothelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 292, L396–L404 (2007).
Mracsko, E. et al. Leukocyte invasion of the brain after experimental intracerebral hemorrhage in mice. Stroke 45, 2107–2114 (2014).
Rolland, W. B. et al. Fingolimod reduces cerebral lymphocyte infiltration in experimental models of rodent intracerebral hemorrhage. Exp. Neurol. 241, 45–55 (2013).
Xi, G. H., Keep, R. F. & Hoff, J. T. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J. Neurosurg. 89, 991–996 (1998).
Lee, K. R. et al. Edema from intracerebral hemorrhage: the role of thrombin. J. Neurosurg. 84, 91–96 (1996).
Sun, Z. et al. Recombinant hirudin treatment modulates aquaporin-4 and aquaporin-9 expression after intracerebral hemorrhage in vivo. Mol. Biol. Rep. 36, 1119–1127 (2009).
Gebel, J. M. et al. Decreased perihematomal edema in thrombolysis-related intracerebral hemorrhage compared with spontaneous intracerebral hemorrhage. Stroke 31, 596–600 (2000).
Levine, J. M. et al. Early edema in warfarin-related intracerebral hemorrhage. Neurocrit. Care 7, 58–63 (2007).
Ducruet, A. F. et al. The complement cascade as a therapeutic target in intracerebral hemorrhage. Exp. Neurol. 219, 398–403 (2009).
Hua, Y., Xi, G., Keep, R. F. & Hoff, J. T. Complement activation in the brain after experimental intracerebral hemorrhage. J. Neurosurg. 92, 1016–1022 (2000).
Garrett, M. C. et al. Synergistic neuroprotective effects of C3a and C5a receptor blockade following intracerebral hemorrhage. Brain Res. 1298, 171–177 (2009).
Huang, F. P. et al. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J. Neurosurg. 96, 287–293 (2002).
Nakamura, T. et al. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J. Neurosurg. 100, 672–678 (2004).
Xie, Q. et al. Deferoxamine attenuates white matter injury in a piglet intracerebral hemorrhage model. Stroke 45, 290–292 (2014).
Lou, M., Lieb, K. & Selim, M. The relationship between hematoma iron content and perihematoma edema: an MRI study. Cerebrovasc. Dis. 27, 266–271 (2009).
Wagner, I. et al. Radiopacity of intracerebral hemorrhage correlates with perihemorrhagic edema. Eur. J. Neurol. 19, 525–528 (2012).
Katsu, M. et al. Hemoglobin-induced oxidative stress contributes to matrix metalloproteinase activation and blood–brain barrier dysfunction in vivo. J. Cereb. Blood Flow Metab. 30, 1939–1950 (2010).
Florczak-Rzepka, M., Grond-Ginsbach, C., Montaner, J. & Steiner, T. Matrix metalloproteinases in human spontaneous intracerebral hemorrhage: an update. Cerebrovasc. Dis. 34, 249–262 (2012).
Zhou, H. J. et al. Thrombin-triggered angiogenesis in rat brains following experimental intracerebral hemorrhage. J. Neurosurg. 117, 920–928 (2012).
Liu, D. Z. et al. Blood–brain barrier breakdown and repair by Src after thrombin-induced injury. Ann. Neurol. 67, 526–533 (2010).
Gebel, J. M. Jr et al. Natural history of perihematomal edema in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke 33, 2631–2635 (2002).
Mould, W. A. et al. Minimally invasive surgery plus recombinant tissue-type plasminogen activator for intracerebral hemorrhage evacuation decreases perihematomal edema. Stroke 44, 627–634 (2013).
McCarron, M. O., McCarron, P. & Alberts, M. J. Location characteristics of early perihaematomal oedema. J. Neurol. Neurosurg. Psychiatry 77, 378–380 (2006).
Wagner, I. et al. Sex differences in perihemorrhagic edema evolution after spontaneous intracerebral hemorrhage. Eur. J. Neurol. 19, 1477–1481 (2012).
Gu, Y., Xi, G., Liu, W., Keep, R. F. & Hua, Y. Estrogen reduces iron-mediated brain edema and neuronal death. Acta Neurochir. Suppl. 106, 159–162 (2010).
Sykora, M., Diedler, J., Turcani, P., Rupp, A. & Steiner, T. Subacute perihematomal edema in intracerebral hemorrhage is associated with impaired blood pressure regulation. J. Neurol. Sci. 284, 108–112 (2009).
James, M. L., Blessing, R., Bennett, E. & Laskowitz, D. T. Apolipoprotein E modifies neurological outcome by affecting cerebral edema but not hematoma size after intracerebral hemorrhage in humans. J. Stroke Cerebrovasc. Dis. 18, 144–149 (2009).
McCarron, M. O. et al. Intracerebral hemorrhage outcome: apolipoprotein E genotype, hematoma, and edema volumes. Neurology 53, 2176–2179 (1999).
Biffi, A. et al. Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann. Neurol. 68, 934–943 (2010).
Qureshi, A. I. et al. Effect of systolic blood pressure reduction on hematoma expansion, perihematomal edema, and 3-month outcome among patients with intracerebral hemorrhage: results from the antihypertensive treatment of acute cerebral hemorrhage study. Arch. Neurol. 67, 570–576 (2010).
Vemmos, K. N. et al. Association between 24-h blood pressure monitoring variables and brain oedema in patients with hyperacute stroke. J. Hypertens. 21, 2167–2173 (2003).
Sansing, L. H., Messe, S. R., Cucchiara, B. L., Lyden, P. D. & Kasner, S. E. Anti-adrenergic medications and edema development after intracerebral hemorrhage. Neurocrit. Care 14, 395–400 (2011).
Mayer, S. A. et al. Perilesional blood flow and edema formation in acute intracerebral hemorrhage: a SPECT study. Stroke 29, 1791–1798 (1998).
Deegan, B. M. et al. Elderly women regulate brain blood flow better than men do. Stroke 42, 1988–1993 (2011).
Naval, N. S. et al. An association of prior statin use with decreased perihematomal edema. Neurocrit. Care 8, 13–18 (2008).
Qureshi, A. I. et al. Association of serum glucose concentrations during acute hospitalization with hematoma expansion, perihematomal edema, and three month outcome among patients with intracerebral hemorrhage. Neurocrit. Care 15, 428–435 (2011).
Allen, C. L. & Bayraktutan, U. Antioxidants attenuate hyperglycaemia-mediated brain endothelial cell dysfunction and blood–brain barrier hyperpermeability. Diabetes Obes. Metab. 11, 480–490 (2009).
Song, E. C. et al. Hyperglycemia exacerbates brain edema and perihematomal cell death after intracerebral hemorrhage. Stroke 34, 2215–2220 (2003).
Feng, W., Tauhid, S., Goel, S., Sidorov, E. V. & Selim, M. Hyperglycemia and outcome in intracerebral hemorrhage: from bedside to bench-more study is needed. Transl. Stroke Res. 3 (Suppl. 1), 113–118 (2012).
Staykov, D. et al. Mild prolonged hypothermia for large intracerebral hemorrhage. Neurocrit. Care 18, 178–183 (2013).
Kawai, N., Kawanishi, M., Okauchi, M. & Nagao, S. Effects of hypothermia on thrombin-induced brain edema formation. Brain Res. 895, 50–58 (2001).
Sun, H., Tang, Y., Guan, X., Li, L. & Wang, D. Effects of selective hypothermia on blood–brain barrier integrity and tight junction protein expression levels after intracerebral hemorrhage in rats. Biol. Chem. 394, 1317–1324 (2013).
Wagner, I. et al. Effects of continuous hypertonic saline infusion on perihemorrhagic edema evolution. Stroke 42, 1540–1545 (2011).
Ryu, J. H. et al. Induced and sustained hypernatremia for the prevention and treatment of cerebral edema following brain injury. Neurocrit. Care 19, 222–231 (2013).
Sansing, L. H. et al. Edema after intracerebral hemorrhage: correlations with coagulation parameters and treatment. J. Neurosurg. 98, 985–992 (2003).
Wang, J. et al. β-adrenoceptor mediated surgery-induced production of pro-inflammatory cytokines in rat microglia cells. J. Neuroimmunol. 223, 77–83 (2010).
Yang, D. et al. Statins protect the blood brain barrier acutely after experimental intracerebral hemorrhage. J. Behav. Brain Sci. 3, 100–106 (2013).
Zazulia, A. R., Diringer, M. N., Derdeyn, C. P. & Powers, W. J. Progression of mass effect after intracerebral hemorrhage. Stroke 30, 1167–1173 (1999).
Walberer, M. et al. Midline-shift corresponds to the amount of brain edema early after hemispheric stroke—an MRI study in rats. J. Neurosurg. Anesthesiol. 19, 105–110 (2007).
Zazulia, A. R., Videen, T. O., Diringer, M. N. & Powers, W. J. Poor correlation between perihematomal MRI hyperintensity and brain swelling after intracerebral hemorrhage. Neurocrit. Care 15, 436–441 (2011).
Volbers, B. et al. Semi-automatic volumetric assessment of perihemorrhagic edema with computed tomography. Eur. J. Neurol. 18, 1323–1328 (2011).
Gebel, J. M. Jr et al. Relative edema volume is a predictor of outcome in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke 33, 2636–2641 (2002).
Staykov, D. et al. Natural course of perihemorrhagic edema after intracerebral hemorrhage. Stroke 42, 2625–2629 (2011).
Sun, W. et al. Predictors of late neurological deterioration after spontaneous intracerebral hemorrhage. Neurocrit. Care 19, 299–305 (2013).
Romero, J. M. et al. Spot sign score predicts rapid bleeding in spontaneous intracerebral hemorrhage. Emerg. Radiol. 19, 195–202 (2012).
Lee, S. H. et al. Effects of celecoxib on hematoma and edema volumes in primary intracerebral hemorrhage: a multicenter randomized controlled trial. Eur. J. Neurol. 20, 1161–1169 (2013).
Chu, K. et al. Celecoxib induces functional recovery after intracerebral hemorrhage with reduction of brain edema and perihematomal cell death. J. Cereb. Blood Flow Metab. 24, 926–933 (2004).
Fu, Y. et al. Fingolimod for the treatment of intracerebral hemorrhage: a 2-arm proof-of-concept study. JAMA Neurol. 71, 1092–1101 (2014).
Yeatts, S. D., Palesch, Y. Y., Moy, C. S. & Selim, M. High dose deferoxamine in intracerebral hemorrhage (HI-DEF) trial: rationale, design, and methods. Neurocrit. Care 19, 257–266 (2013).
Gu, Y., Hua, Y., Keep, R. F., Morgenstern, L. B. & Xi, G. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke 40, 2241–2243 (2009).
Okauchi, M. et al. Deferoxamine treatment for intracerebral hemorrhage in aged rats: therapeutic time window and optimal duration. Stroke 41, 375–382 (2010).
Gonzales, N. R. et al. Design of a prospective, dose-escalation study evaluating the Safety of Pioglitazone for Hematoma Resolution in Intracerebral Hemorrhage (SHRINC). Int. J. Stroke 8, 388–396 (2013).
Zhao, X. R. et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor γ in microglia/macrophages. Ann. Neurol. 61, 352–362 (2007).
Rincon, F., Friedman, D. P., Bell, R., Mayer, S. A. & Bray, P. F. Targeted temperature management after intracerebral hemorrhage (TTM-ICH): methodology of a prospective randomized clinical trial. Int. J. Stroke 9, 646–651 (2014).
Kollmar, R. et al. Cooling in intracerebral hemorrhage (CINCH) trial: protocol of a randomized German–Austrian clinical trial. Int. J. Stroke 7, 168–172 (2012).
Acknowledgements
S.U. is supported by the Leon Rosenberg, MD Medical Student Research Fund in Genetics (Yale University School of Medicine) and a 2014 Student Scholarship in Cerebrovascular Disease and Stroke (American Heart Association Stroke Council). L.A.B. is supported by the National Institute of Neurological Disorders and Stroke (NINDS; K12-NS049453). J.M.S. is supported by grants from the Department of Veterans Affairs (Baltimore; BX001629), the NINDS (NS060801, NS061808), and the National Heart, Lung and Blood Institute (HL082517).
Author information
Authors and Affiliations
Contributions
S.U. and K.N.S. originated the overall concept for this Review. All authors contributed to several drafts of the article and provided important intellectual exchanges during formulation of key concepts. S.U., J.M.S. and K.N.S. contributed to every draft and supplied figures. A.O.V. was responsible for procuring autopsy specimens, and J.M.S. was responsible for SUR1 detection in intracerebral haemorrhage tissue shown in Figure 3.
Corresponding author
Ethics declarations
Competing interests
W.T.K., and L.A.B. and K.N.S. are investigators in GAMES-RP, a phase II study of an investigational compound aimed at preventing swelling after large stroke. J.M.S. holds a US patent (7,285,574, Methods for treating neural cell swelling). The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Urday, S., Kimberly, W., Beslow, L. et al. Targeting secondary injury in intracerebral haemorrhage—perihaematomal oedema. Nat Rev Neurol 11, 111–122 (2015). https://doi.org/10.1038/nrneurol.2014.264
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2014.264
This article is cited by
-
Mesenchymal stem cells transplantation combined with IronQ attenuates ICH-induced inflammation response via Mincle/syk signaling pathway
Stem Cell Research & Therapy (2023)
-
Self-healing hydrogel as an injectable implant: translation in brain diseases
Journal of Biomedical Science (2023)
-
Peak Edema Extension Distance: An Edema Measure Independent from Hematoma Volume Associated with Functional Outcome in Intracerebral Hemorrhage
Neurocritical Care (2023)
-
Ion Channel Dysregulation Following Intracerebral Hemorrhage
Neuroscience Bulletin (2023)
-
Deubiquitylating Enzyme OTUB1 Facilitates Neuronal Survival After Intracerebral Hemorrhage Via Inhibiting NF-κB-triggered Apoptotic Cascades
Molecular Neurobiology (2023)