Key Points
-
Nonmotor symptoms, particularly hyposmia and constipation, precede the motor symptoms of Parkinson disease (PD) by many years, and the lifetime risk of PD negatively correlates with the frequency of bowel movements
-
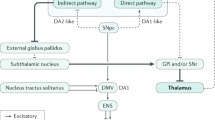
Neurodegeneration in PD seems to start in the olfactory bulb, the enteric nervous system, the dorsal motor nucleus of the vagus nerve and the intermediolateral nucleus in the thoracic and sacral spinal cord
-
Evidence suggests that environmental factors have an important role in triggering and/or propagating the pathology of PD; the olfactory and gastrointestinal systems are gateways to the environment
-
Chronic intragastral administration of rotenone in mice has resulted in neuropathology and symptoms typical of PD that developed in a chronological and regional sequence that corresponds to the Braak staging system
-
PD pathology that involves α-synuclein spreads from the enteric nervous system to the CNS by trans-synaptic cell-to-cell transmission in intact sympathetic and parasympathetic nerves
-
α-Synuclein impairs mitochondrial activity and causes oxidative stress in neurons, especially dopaminergic neurons in the substantia nigra and noradrenergic neurons in the locus coeruleus
Abstract
Parkinson disease (PD) follows a defined clinical pattern, and a range of nonmotor symptoms precede the motor phase. The predominant early nonmotor manifestations are olfactory impairment and constipation. The pathology that accompanies these symptoms is consistent with the Braak staging system: α-synuclein in the dorsal motor nucleus of the vagus nerve, the olfactory bulb, the enteric nervous system (ENS) and the submandibular gland, each of which is a gateway to the environment. The neuropathological process that leads to PD seems to start in the ENS or the olfactory bulb and spreads via rostrocranial transmission to the substantia nigra and further into the CNS, raising the intriguing possibility that environmental substances can trigger pathogenesis. Evidence from epidemiological studies and animal models supports this hypothesis. For example, in mice, intragastric administration of the pesticide rotenone can almost completely reproduce the typical pathological and clinical features of PD. In this Review, we present clinical and pathological evidence to support the hypothesis that PD starts in the gut and spreads via trans-synaptic cell-to-cell transfer of pathology through the sympathetic and parasympathetic nervous systems to the substantia nigra and the CNS. We also consider how environmental factors might trigger pathogenesis, and the potential for therapeutically targeting the mechanisms of these initial stages.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hughes, A. J., Daniel, S. E., Kilford, L. & Lees, A. J. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 55, 181–184 (1992).
Haehner, A., Hummel, T. & Reichmann, H. Olfactory dysfunction as a diagnostic marker for Parkinson's disease. Expert Rev. Neurother. 9, 1773–1779 (2009).
Iranzo, A. et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 5, 572–577 (2006).
Abbott, R. D. et al. Frequency of bowel movements and the future risk of Parkinson's disease. Neurology 57, 456–462 (2001).
Jost, W. H. Gastrointestinal dysfunction in Parkinson's disease. J. Neurol. Sci. 289, 69–73 (2010).
Savica, R. et al. Medical records documentation of constipation preceding Parkinson disease: a case–control study. Neurology 73, 1752–1758 (2009).
Cersosimo, M. G. et al. Gastrointestinal manifestations in Parkinson's disease: prevalence and occurrence before motor symptoms. J. Neurol. 260, 1332–1338 (2013).
Reichmann, H., Schneider, C. & Löhle, M. Non-motor features of Parkinson's disease: depression and dementia. Parkinsonism Relat. Disord. 15 (Suppl. 3), S87–S92 (2009).
Schrag, A., Jahanshahi, M. & Quinn, N. How does Parkinson's disease affect quality of life? A comparison with quality of life in the general population. Mov. Disord. 15, 1112–1118 (2000).
Martinez-Martin, P., Rodriguez-Blazquez, C., Kurtis, M. M. & Chaudhuri, K. R. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson's disease. Mov. Disord. 26, 399–406 (2011).
Müller, B., Assmus, J., Herlofson, K., Larsen, J. P. & Tysnes, O. B. Importance of motor vs. non-motor symptoms for health-related quality of life in early Parkinson's disease. Parkinsonism Relat. Disord. 19, 1027–1032 (2013).
O'Sullivan, S. S. et al. Nonmotor symptoms as presenting complaints in Parkinson's disease: a clinicopathological study. Mov. Disord. 23, 101–106 (2008).
Gallagher, D. A., Lees, A. J. & Schrag, A. What are the most important nonmotor symptoms in patients with Parkinson's disease and are we missing them? Mov. Disord. 25, 2493–2500 (2010).
Gibb, W. R. & Lees, A. J. Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 54, 388–396 (1991).
Goedert, M., Spillantini, M. G., Del Tredici, K. & Braak, H. 100 years of Lewy pathology. Nat. Rev. Neurol. 9, 13–24 (2013).
Halliday, G., McCann, H. & Shepherd, C. Evaluation of the Braak hypothesis: how far can it explain the pathogenesis of Parkinson's disease? Expert Rev. Neurother. 12, 673–686 (2012).
Reichmann, H. View point: etiology in Parkinson's disease. Dual hit or spreading intoxication. J. Neurol. Sci. 310, 9–11 (2011).
Pfeiffer, R. F. Gastrointestinal dysfunction in Parkinson's disease. Lancet Neurol. 2, 107–116 (2003).
Martinez-Martin, P. The importance of non-motor disturbances to quality of life in Parkinson's disease. J. Neurol. Sci. 310, 12–16 (2011).
Rodríguez-Violante, M., Cervantes-Arriaga, A., Villar-Velarde, A. & Corona, T. Prevalence of non-motor dysfunction among Parkinson's disease patients from a tertiary referral center in Mexico City. Clin. Neurol. Neurosurg. 112, 883–885 (2010).
Stern, M. B. & Siderowf, A. Parkinson's at risk syndrome: can Parkinson's disease be predicted? Mov. Disord. 25 (Suppl. 1), S89–S93 (2010).
Lang, A. E. A critical appraisal of the premotor symptoms of Parkinson's disease: potential usefulness in early diagnosis and design of neuroprotective trials. Mov. Disord. 26, 775–783 (2011).
Edwards, L. L., Pfeiffer, R. F., Quigley, E. M., Hofman, R. & Balluff, M. Gastrointestinal symptoms in Parkinson's disease. Mov. Disord. 6, 151–156 (1991).
Kaye, J., Gage, H., Kimber, A., Storey, L. & Trend, P. Excess burden of constipation in Parkinson's disease: a pilot study. Mov. Disord. 21, 1270–1273 (2006).
Chaudhuri, K. R., Healy, D. G. & Schapira, A. H. Non-motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol. 5, 235–245 (2006).
Verbaan, D. et al. Patient-reported autonomic symptoms in Parkinson disease. Neurology 69, 333–341 (2007).
Goetze, O. et al. Predictors of gastric emptying in Parkinson's disease. Neurogastroenterol. Motil. 18, 369–375 (2006).
Chaudhuri, K. R. et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson's disease: the NMSQuest study. Mov. Disord. 21, 916–923 (2006).
Martinez-Martin, P. et al. Prevalence of nonmotor symptoms in Parkinson's disease in an international setting; study using nonmotor symptoms questionnaire in 545 patients. Mov. Disord. 22, 1623–1629 (2007).
Pfeiffer, R. F. Gastrointestinal dysfunction in Parkinson's disease. Parkinsonism Relat. Disord. 17, 10–15 (2011).
Jost, W. H. Gastrointestinal motility problems in patients with Parkinson's disease. Effects of antiparkinsonian treatment and guidelines for management. Drugs Aging 10, 249–258 (1997).
Barone, P. et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson's disease. Mov. Disord. 24, 1641–1649 (2009).
Korczyn, A. D. Autonomic nervous system disturbances in Parkinson's disease. Adv. Neurol. 53, 463–468 (1990).
Ashraf, W., Pfeiffer, R. F., Park, F., Lof, J. & Quigley, E. M. Constipation in Parkinson's disease: objective assessment and response to psyllium. Mov. Disord. 12, 946–951 (1997).
Adams-Carr, K. L. et al. Constipation preceding Parkinson's disease: a systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry (2015).
Cersosimo, M. G. & Benarroch, E. E. Neural control of the gastrointestinal tract: implications for Parkinson disease. Mov Disord. 23, 1065–1075 (2008).
Jost, W. H. & Schrank, B. Defecatory disorders in de novo Parkinsonians—colonic transit and electromyogram of the external anal sphincter. Wien Klin. Wochenschr. 110, 535–537 (1998).
Sakakibara, R. et al. Colonic transit time and rectoanal videomanometry in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 74, 268–272 (2003).
Sakakibara, R. et al. Bladder, bowel, and sexual dysfunction in Parkinson's disease. Parkinsons Dis. 2011, 924605 (2011).
Wang, C. P., Sung, W. H., Wang, C. C. & Tsai, P. Y. Early recognition of pelvic floor dyssynergia and colorectal assessment in Parkinson's disease associated with bowel dysfunction. Colorectal Dis. 15, e130–e137 (2013).
Cersosimo, M. G. & Benarroch, E. E. Pathological correlates of gastrointestinal dysfunction in Parkinson's disease. Neurobiol. Dis. 46, 559–564 (2012).
Edwards, L. L., Quigley, E. M., Harned, R. K., Hofman, R. & Pfeiffer, R. F. Characterization of swallowing and defecation in Parkinson's disease. Am. J. Gastroenterol. 89, 15–25 (1994).
Jost, W. H. & Schimrigk, K. Constipation in Parkinson's disease. Klin. Wochenschr. 69, 906–909 (1991).
Edwards, L. L., Quigley, E. M. & Pfeiffer, R. F. Gastrointestinal dysfunction in Parkinson's disease: frequency and pathophysiology. Neurology 42, 726–732 (1992).
Bassotti, G. et al. Manometric investigation of anorectal function in early and late stage Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 68, 768–770 (2000).
Mathers, S. E., Kempster, P. A., Swash, M. & Lees, A. J. Constipation and paradoxical puborectalis contraction in anismus and Parkinson's disease: a dystonic phenomenon? J. Neurol. Neurosurg. Psychiatry 51, 1503–1507 (1988).
Ashraf, W., Pfeiffer, R. F. & Quigley, E. M. Anorectal manometry in the assessment of anorectal function in Parkinson's disease: a comparison with chronic idiopathic constipation. Mov. Disord. 9, 655–663 (1994).
Jost, W. H., Schrank, B., Herold, A. & Leiss, O. Functional outlet obstruction: anismus, spastic pelvic floor syndrome, and dyscoordination of the voluntary sphincter muscles. Definition, diagnosis, and treatment from the neurologic point of view. Scand. J. Gastroenterol. 34, 449–453 (1999).
Spillantini, M. G. et al. α-Synuclein in Lewy bodies. Nature 388, 839–840 (1997).
Gaspar, P. & Gray, F. Dementia in idiopathic Parkinson's disease. A neuropathological study of 32 cases. Acta Neuropathol. 64, 43–52 (1984).
Braak, H. et al. Pattern of brain destruction in Parkinson's and Alzheimer's diseases. J. Neural Transm. 103, 455–490 (1996).
Braak, H. et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging 24, 197–211 (2003).
Braak, H., Ghebremedhin, E., Rub, U., Bratzke, H. & Del Tredici, K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 318, 121–134 (2004).
Del Tredici, K., Rub, U., De Vos, R. A., Bohl, J. R. & Braak, H. Where does parkinson disease pathology begin in the brain? J. Neuropathol. Exp. Neurol. 61, 413–426 (2002).
Braak, H. & Del Tredici, K. Neuroanatomy and pathology of sporadic Parkinson's disease. Adv. Anat. Embryol. Cell Biol. 201, 1–119 (2009).
Braak, H., Rub, U., Gai, W. P. & Del Tredici, K. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. 110, 517–536 (2003).
Hawkes, C. H., Del Tredici, K. & Braak, H. Parkinson's disease: a dual-hit hypothesis. Neuropathol. Appl. Neurobiol. 33, 599–614 (2007).
Beach, T. G. et al. Olfactory bulb α-synucleinopathy has high specificity and sensitivity for Lewy body disorders. Acta Neuropathol. 117, 169–174 (2009).
Braak, H., de Vos, R. A., Bohl, J. & Del Tredici, K. Gastric α-synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease-related brain pathology. Neurosci. Lett. 396, 67–72 (2006).
Del Tredici, K., Hawkes, C. H., Ghebremedhin, E. & Braak, H. Lewy pathology in the submandibular gland of individuals with incidental Lewy body disease and sporadic Parkinson's disease. Acta Neuropathol. 119, 703–713 (2010).
Orimo, S. et al. Cardiac sympathetic denervation precedes neuronal loss in the sympathetic ganglia in Lewy body disease. Acta Neuropathol. 109, 583–588 (2005).
Minguez-Castellanos, A. et al. Do α-synuclein aggregates in autonomic plexuses predate Lewy body disorders?: a cohort study. Neurology 68, 2012–2018 (2007).
Wakabayashi, K., Takahashi, H., Ohama, E. & Ikuta, F. Parkinson's disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol. 79, 581–583 (1990).
Braak, H., Sastre, M., Bohl, J. R., de Vos, R. A. & Del Tredici, K. Parkinson's disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol. 113, 421–429 (2007).
Wakabayashi, K., Takahashi, H., Takeda, S., Ohama, E. & Ikuta, F. Parkinson's disease: the presence of Lewy bodies in Auerbach's and Meissner's plexuses. Acta Neuropathol. 76, 217–221 (1988).
Kupsky, W. J., Grimes, M. M., Sweeting, J., Bertsch, R. & Cote, L. J. Parkinson's disease and megacolon: concentric hyaline inclusions (Lewy bodies) in enteric ganglion cells. Neurology 37, 1253–1255 (1987).
Lebouvier, T. et al. Pathological lesions in colonic biopsies during Parkinson's disease. Gut 57, 1741–1743 (2008).
Qualman, S. J., Haupt, H. M., Yang, P. & Hamilton, S. R. Esophageal Lewy bodies associated with ganglion cell loss in achalasia. Similarity to Parkinson's disease. Gastroenterology 87, 848–856 (1984).
Abbott, R. D. et al. Bowel movement frequency in late-life and incidental Lewy bodies. Mov. Disord. 22, 1581–1586 (2007).
Petrovitch, H. et al. Bowel movement frequency in late-life and substantia nigra neuron density at death. Mov. Disord. 24, 371–376 (2009).
Del Tredici, K. & Braak, H. Spinal cord lesions in sporadic Parkinson's disease. Acta Neuropathol. 124, 643–664 (2012).
Bloch, A., Probst, A., Bissig, H., Adams, H. & Tolnay, M. α-Synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol. Appl. Neurobiol. 32, 284–295 (2006).
Albin, R. L., Young, A. B. & Penney, J. B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–375 (1989).
Klingelhoefer, L. & Reichmann, H. Dementia—the real problem for patients with Parkinson's disease Basal Ganglia 4, 9–13 (2014).
Kalaitzakis, M. E., Graeber, M. B., Gentleman, S. M. & Pearce, R. K. The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson's disease: a critical analysis of α-synuclein staging. Neuropathol. Appl. Neurobiol. 34, 284–295 (2008).
Attems, J. & Jellinger, K. A. The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson's disease. Neuropathol. Appl. Neurobiol. 34, 466–467 (2008).
Zaccai, J., Brayne, C., McKeith, I., Matthews, F. & Ince, P. G. Patterns and stages of α-synucleinopathy: Relevance in a population-based cohort. Neurology 70, 1042–1048 (2008).
Parkkinen, L., Pirttila, T. & Alafuzoff, I. Applicability of current staging/categorization of α-synuclein pathology and their clinical relevance. Acta Neuropathol. 115, 399–407 (2008).
Jellinger, K. A. Lewy body-related α-synucleinopathy in the aged human brain. J. Neural Transm. 111, 1219–1235 (2004).
Jellinger, K. A. α-Synuclein pathology in Parkinson's and Alzheimer's disease brain: incidence and topographic distribution--a pilot study. Acta Neuropathol. 106, 191–201 (2003).
Beach, T. G. et al. Multi-organ distribution of phosphorylated α-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 119, 689–702 (2010).
Gelpi, E. et al. Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Mov. Disord. 29, 1010–1018 (2014).
Annerino, D. M. et al. Parkinson's disease is not associated with gastrointestinal myenteric ganglion neuron loss. Acta Neuropathol. 124, 665–680 (2012).
Lebouvier, T. et al. Colonic biopsies to assess the neuropathology of Parkinson's disease and its relationship with symptoms. PLoS ONE 5, e12728 (2010).
De Giorgio, R. et al. Enteric glia and neuroprotection: basic and clinical aspects. Am. J. Physiol. Gastrointest Liver Physiol. 303, G887–G893 (2012).
Devos, D. et al. Colonic inflammation in Parkinson's disease. Neurobiol. Dis. 50, 42–48 (2013).
Clairembault, T. et al. Enteric GFAP expression and phosphorylation in Parkinson's disease. J. Neurochem. 130, 805–815 (2014).
Bassotti, G. et al. The role of glial cells and apoptosis of enteric neurones in the neuropathology of intractable slow transit constipation. Gut 55, 41–46 (2006).
Forsyth, C. B. et al. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson's disease. PLoS ONE 6, e28032 (2011).
Clairembault, T., Leclair-Visonneau, L., Neunlist, M. & Derkinderen, P. Enteric glial cells: new players in Parkinson's disease? Mov. Disord. 30, 494–498 (2015).
Singaram, C. et al. Dopaminergic defect of enteric nervous system in Parkinson's disease patients with chronic constipation. Lancet 346, 861–864 (1995).
Parkkinen, L., Soininen, H., Laakso, M. & Alafuzoff, I. α-Synuclein pathology is highly dependent on the case selection. Neuropathol. Appl. Neurobiol. 27, 314–325 (2001).
Saito, Y. et al. Lewy body-related α-synucleinopathy in aging. J. Neuropathol. Exp. Neurol. 63, 742–749 (2004).
Lai, B. C., Marion, S. A., Teschke, K. & Tsui, J. K. Occupational and environmental risk factors for Parkinson's disease. Parkinsonism Relat. Disord. 8, 297–309 (2002).
Priyadarshi, A., Khuder, S. A., Schaub, E. A. & Priyadarshi, S. S. Environmental risk factors and Parkinson's disease: a metaanalysis. Environ. Res. 86, 122–127 (2001).
Gorell, J. M., Johnson, C. C., Rybicki, B. A., Peterson, E. L. & Richardson, R. J. The risk of Parkinson's disease with exposure to pesticides, farming, well water, and rural living. Neurology 50, 1346–1350 (1998).
Gatto, N. M., Cockburn, M., Bronstein, J., Manthripragada, A. D. & Ritz, B. Well-water consumption and Parkinson's disease in rural California. Environ. Health Perspect. 117, 1912–1918 (2009).
Seidler, A. et al. Possible environmental, occupational, and other etiologic factors for Parkinson's disease: a case–control study in Germany. Neurology 46, 1275–1284 (1996).
Fleming, L., Mann, J. B., Bean, J., Briggle, T. & Sanchez-Ramos, J. R. Parkinson's disease and brain levels of organochlorine pesticides. Ann. Neurol. 36, 100–103 (1994).
Semchuk, K. M., Love, E. J. & Lee, R. G. Parkinson's disease and exposure to agricultural work and pesticide chemicals. Neurology 42, 1328–1335 (1992).
Manning-Bog, A. B. et al. The herbicide paraquat causes up-regulation and aggregation of α-synuclein in mice: paraquat and α-synuclein. J. Biol. Chem. 277, 1641–1644 (2002).
Huang, C. C. et al. Progression after chronic manganese exposure. Neurology 43, 1479–1483 (1993).
Tanner, C. M. et al. Rotenone, paraquat, and Parkinson's disease. Environ. Health Perspect. 119, 866–872 (2011).
Baron, J. A. Cigarette smoking and Parkinson's disease. Neurology 36, 1490–1496 (1986).
Ascherio, A. et al. Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann. Neurol. 50, 56–63 (2001).
Derkinderen, P., Shannon, K. M. & Brundin, P. Gut feelings about smoking and coffee in Parkinson's disease. Mov. Disord. 29, 976–979 (2014).
Scheperjans, F., Pekkonen, E., Kaakkola, S. & Auvinen, P. Linking smoking, coffee, urate, and Parkinson's disease—a role for gut microbiota? J. Parkinsons Dis. (2015).
Scheperjans, F. et al. Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov. Disord. 30, 350–358 (2015).
Forsythe, P. & Kunze, W. A. Voices from within: gut microbes and the CNS. Cell. Mol. Life Sci. 70, 55–69 (2013).
Cryan, J. F. & Dinan, T. G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 13, 701–712 (2012).
Marras, C. & Lang, A. Parkinson's disease subtypes: lost in translation? J. Neurol. Neurosurg. Psychiatry 84, 409–415 (2013).
van Rooden, S. M. et al. Clinical subtypes of Parkinson's disease. Mov. Disord. 26, 51–58 (2011).
Ulusoy, A. et al. Caudo-rostral brain spreading of α-synuclein through vagal connections. EMBO Mol. Med. 5, 1051–1059 (2013).
Perez-Burgos, A. et al. Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. Am. J. Physiol. Gastrointest Liver Physiol. 304, G211–G220 (2013).
Bravo, J. A. et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl Acad. Sci. USA 108, 16050–16055 (2011).
Svensson, E. et al. Vagotomy and subsequent risk of Parkinson's disease. Ann. Neurol. 78, 522–529 (2015).
Tan, A. H. et al. Small intestinal bacterial overgrowth in Parkinson's disease. Parkinsonism Relat. Disord. 20, 535–540 (2014).
Fasano, A. et al. The role of small intestinal bacterial overgrowth in Parkinson's disease. Mov. Disord. 28, 1241–1249 (2013).
Tan, A. H. et al. Helicobacter pylori infection is associated with worse severity of Parkinson's disease. Parkinsonism Relat. Disord. 21, 221–225 (2015).
Rees, K. et al. Helicobacter pylori eradication for Parkinson's disease. Cochrane Database of Systematic Reviews, Issue 11 Art. No.: CD008453. http://dx.doi.org/10.1002/14651858.CD008453.pub2.
Lee, W. Y., Yoon, W. T., Shin, H. Y., Jeon, S. H. & Rhee, P. L. Helicobacter pylori infection and motor fluctuations in patients with Parkinson's disease. Mov. Disord. 23, 1696–1700 (2008).
Narozanska, E. et al. Pharmacokinetics of levodopa in patients with Parkinson disease and motor fluctuations depending on the presence of Helicobacter pylori infection. Clin. Neuropharmacol. 37, 96–99 (2014).
Logroscino, G. et al. Dietary lipids and antioxidants in Parkinson's disease: a population-based, case–control study. Ann. Neurol. 39, 89–94 (1996).
Anderson, C. et al. Dietary factors in Parkinson's disease: the role of food groups and specific foods. Mov. Disord. 14, 21–27 (1999).
Abbott, R. D. et al. Environmental, life-style, and physical precursors of clinical Parkinson's disease: recent findings from the Honolulu–Asia Aging Study. J. Neurol. 250 (Suppl. 3), III30–III39 (2003).
Chen, H., Zhang, S. M., Hernan, M. A., Willett, W. C. & Ascherio, A. Dietary intakes of fat and risk of Parkinson's disease. Am. J. Epidemiol. 157, 1007–1014 (2003).
de Lau, L. M. et al. Dietary fatty acids and the risk of Parkinson disease: the Rotterdam study. Neurology 64, 2040–2045 (2005).
Powers, K. M. et al. Dietary fats, cholesterol and iron as risk factors for Parkinson's disease. Parkinsonism Relat. Disord. 15, 47–52 (2009).
Miyake, Y. et al. Dietary fat intake and risk of Parkinson's disease: a case-control study in Japan. J. Neurol. Sci. 288, 117–122 (2010).
Kamel, F. et al. Dietary fat intake, pesticide use, and Parkinson's disease. Parkinsonism Relat. Disord. 20, 82–87 (2014).
Bove, J., Prou, D., Perier, C. & Przedborski, S. Toxin-induced models of Parkinson's disease. NeuroRx 2, 484–494 (2005).
McDowell, K. & Chesselet, M. F. Animal models of the non-motor features of Parkinson's disease. Neurobiol. Dis. 46, 597–606 (2012).
Hisahara, S. & Shimohama, S. Toxin-induced and genetic animal models of Parkinson's disease. Parkinsons Dis. 2011, 951709 (2010).
Betarbet, R. et al. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat. Neurosci. 3, 1301–1306 (2000).
Hoglinger, G. U. et al. Chronic systemic complex I inhibition induces a hypokinetic multisystem degeneration in rats. J. Neurochem. 84, 491–502 (2003).
Rojo, A. I., Cavada, C., de Sagarra, M. R. & Cuadrado, A. Chronic inhalation of rotenone or paraquat does not induce Parkinson's disease symptoms in mice or rats. Exp. Neurol. 208, 120–126 (2007).
Pan-Montojo, F. et al. Progression of Parkinson's disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS ONE 5, e8762 (2010).
Inden, M. et al. Neurodegeneration of mouse nigrostriatal dopaminergic system induced by repeated oral administration of rotenone is prevented by 4-phenylbutyrate, a chemical chaperone. J. Neurochem. 101, 1491–1504 (2007).
Tasselli, M. et al. Effects of oral administration of rotenone on gastrointestinal functions in mice. Neurogastroenterol. Motil. 25, e183–e193 (2013).
Sherer, T. B., Kim, J. H., Betarbet, R. & Greenamyre, J. T. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and α-synuclein aggregation. Exp. Neurol. 179, 9–16 (2003).
Silva, B. A., Einarsdottir, O., Fink, A. L. & Uversky, V. N. Biophysical characterization of α-synuclein and rotenone interaction. Biomolecules 3, 703–732 (2013).
Yuan, Y. H. et al. The molecular mechanism of rotenone-induced α-synuclein aggregation: emphasizing the role of the calcium/GSK3β pathway. Toxicol. Lett. 233, 163–171 (2015).
Chorfa, A. et al. A variety of pesticides trigger in vitro α-synuclein accumulation, a key event in Parkinson's disease. Arch. Toxicol. http://dx.doi.org/10.1007/s00204-014-1388-2.
Danzer, K. M. et al. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol. Neurodegener. 7, 42 (2012).
Angot, E. et al. Alpha-synuclein cell-to-cell transfer and seeding in grafted dopaminergic neurons in vivo. PLoS ONE 7, e39465 (2012).
Desplats, P. et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of α-synuclein. Proc. Natl Acad. Sci. USA 106, 13010–13015 (2009).
Brundin, P., Li, J. Y., Holton, J. L., Lindvall, O. & Revesz, T. Research in motion: the enigma of Parkinson's disease pathology spread. Nat. Rev. Neurosci. 9, 741–745 (2008).
Li, J. Y. et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat. Med. 14, 501–503 (2008).
Kordower, J. H. & Brundin, P. Lewy body pathology in long-term fetal nigral transplants: is Parkinson's disease transmitted from one neural system to another? Neuropsychopharmacology 34, 254 (2009).
Pan-Montojo, F. et al. Environmental toxins trigger PD-like progression via increased α-synuclein release from enteric neurons in mice. Sci. Rep. 2, 898 (2012).
Ling, E. A., Shieh, J. Y., Wen, C. Y., Yick, T. Y. & Wong, W. C. The dorsal motor nucleus of the vagus nerve of the hamster: ultrastructure of vagal neurons and their responses to vagotomy. J. Anat. 152, 161–172 (1987).
Holmqvist, S. et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 128, 805–820 (2014).
Hardy, J. Expression of normal sequence pathogenic proteins for neurodegenerative disease contributes to disease risk: 'permissive templating' as a general mechanism underlying neurodegeneration. Biochem. Soc. Trans. 33, 578–581 (2005).
Schapira, A. H. Disease modification in Parkinson's disease. Lancet Neurol. 3, 362–368 (2004).
Mizuno, Y. et al. Deficiencies in complex I subunits of the respiratory chain in Parkinson's disease. Biochem. Biophys. Res. Commun. 163, 1450–1455 (1989).
Franco-Iborra, S., Vila, M. & Perier, C. The Parkinson disease mitochondrial hypothesis: where are we at? Neuroscientist http://dx.doi.org/10.1177/1073858415574600.
Jenner, P. Oxidative stress in Parkinson's disease. Ann. Neurol. 53 (Suppl. 3), S26–S36 (2003).
McGeer, P. L. & McGeer, E. G. Inflammation and neurodegeneration in Parkinson's disease. Parkinsonism Relat. Disord. 10 (Suppl. 1), S3–S7 (2004).
Gao, H. M., Hong, J. S., Zhang, W. & Liu, B. Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson's disease. J. Neurosci. 23, 1228–1236 (2003).
Alvarez-Erviti, L. et al. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol. Dis. 42, 360–367 (2011).
Hasegawa, T. et al. The AAA-ATPase VPS4 regulates extracellular secretion and lysosomal targeting of α-synuclein. PLoS ONE 6, e29460 (2011).
Luk, K. C. et al. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953 (2012).
Lee, H. J. et al. Assembly-dependent endocytosis and clearance of extracellular α-synuclein. Int. J. Biochem. Cell Biol. 40, 1835–1849 (2008).
Braidy, N. et al. Alpha-synuclein transmission and mitochondrial toxicity in primary human foetal enteric neurons in vitro. Neurotox. Res. 25, 170–182 (2014).
Esteves, A. R., Arduino, D. M., Silva, D. F., Oliveira, C. R. & Cardoso, S. M. Mitochondrial dysfunction: the road to alpha-synuclein oligomerization in PD. Parkinsons Dis. 2011, 693761 (2011).
Choi, W. S., Palmiter, R. D. & Xia, Z. Loss of mitochondrial complex I activity potentiates dopamine neuron death induced by microtubule dysfunction in a Parkinson's disease model. J. Cell Biol. 192, 873–882 (2011).
Greenamyre, J. T., Betarbet, R. & Sherer, T. B. The rotenone model of Parkinson's disease: genes, environment and mitochondria. Parkinsonism Relat. Disord. 9 (Suppl. 2), S59–S64 (2003).
Klegeris, A. et al. Alpha-synuclein and its disease-causing mutants induce ICAM-1 and IL-6 in human astrocytes and astrocytoma cells. FASEB J. 20, 2000–2008 (2006).
McGeer, P. L. & McGeer, E. G. The α-synuclein burden hypothesis of Parkinson disease and its relationship to Alzheimer disease. Exp. Neurol. 212, 235–238 (2008).
Hunot, S. et al. FcepsilonRII/CD23 is expressed in Parkinson's disease and induces, in vitro, production of nitric oxide and tumor necrosis factor-alpha in glial cells. J. Neurosci. 19, 3440–3447 (1999).
Wagner, J. et al. Anle138b: a novel oligomer modulator for disease-modifying therapy of neurodegenerative diseases such as prion and Parkinson's disease. Acta Neuropathol. 125, 795–813 (2013).
Zhu, M., Han, S. & Fink, A. L. Oxidized quercetin inhibits α-synuclein fibrillization. Biochim. Biophys. Acta 1830, 2872–2881 (2013).
Horvath, I. et al. Modulation of α-synuclein fibrillization by ring-fused 2-pyridones: templation and inhibition involve oligomers with different structure. Arch. Biochem. Biophys. 532, 84–90 (2013).
Shaltiel-Karyo, R. et al. Differential inhibition of α-synuclein oligomeric and fibrillar assembly in parkinson's disease model by cinnamon extract. Biochim. Biophys. Acta 1820, 1628–1635 (2012).
McLean, P. J. et al. TorsinA and heat shock proteins act as molecular chaperones: suppression of α-synuclein aggregation. J. Neurochem. 83, 846–854 (2002).
Du, Y. et al. Histone deacetylase 6 regulates cytotoxic α-synuclein accumulation through induction of the heat shock response. Neurobiol. Aging 35, 2316–2328 (2014).
Faria, C. et al. Inhibition of formation of α-synuclein inclusions by mannosylglycerate in a yeast model of Parkinson's disease. Biochim. Biophys. Acta 1830, 4065–4072 (2013).
Spencer, B. et al. ESCRT-mediated uptake and degradation of brain-targeted α-synuclein single chain antibody attenuates neuronal degeneration in vivo. Mol. Ther. 22, 1753–1767 (2014).
Games, D. et al. Reducing C-terminal-truncated alpha-ynuclein by immunotherapy attenuates neurodegeneration and propagation in Parkinson's disease-like models. J. Neurosci. 34, 9441–9454 (2014).
Besong-Agbo, D. et al. Naturally occurring α-synuclein autoantibody levels are lower in patients with Parkinson disease. Neurology 80, 169–175 (2013).
Lindstrom, V. et al. Immunotherapy targeting α-synuclein protofibrils reduced pathology in (Thy-1)-h[A30P] α-synuclein mice. Neurobiol. Dis. 69, 134–143 (2014).
Tran, H. T. et al. α-Synuclein immunotherapy blocks uptake and templated propagation of misfolded α-synuclein and neurodegeneration. Cell Rep. 7, 2054–2065 (2014).
Menke, T. et al. Coenzyme Q10 reduces the toxicity of rotenone in neuronal cultures by preserving the mitochondrial membrane potential. Biofactors 18, 65–72 (2003).
Storch, A. et al. Randomized, double-blind, placebo-controlled trial on symptomatic effects of coenzyme Q10 in Parkinson disease. Arch. Neurol. 64, 938–944 (2007).
Sandoval-Acuna, C., Ferreira, J. & Speisky, H. Polyphenols and mitochondria: an update on their increasingly emerging ROS-scavenging independent actions. Arch. Biochem. Biophys. 559, 75–90 (2014).
Sakakibara, R. et al. Questionnaire-based assessment of pelvic organ dysfunction in Parkinson's disease. Auton. Neurosci. 92, 76–85 (2001).
Edwards, L., Quigley, E. M., Hofman, R. & Pfeiffer, R. F. Gastrointestinal symptoms in Parkinson disease: 18-month follow-up study. Mov. Disord. 8, 83–86 (1993).
Jost, W. H., Jung, G. & Schimrigk, K. Colonic transit time in nonidiopathic Parkinson's syndrome. Eur. Neurol. 34, 329–331 (1994).
Acknowledgements
The authors are very thankful for the provision of Figure 1c by M. Meinhardt and G. Baretton at Neuropathology, Institute of Pathologie, Technical University Dresden, Germany and of Figure 2b and 2c by W. J. Schulz-Schaeffer at the Prion and Dementia Research Unit, Neuropathology, University Medical Center Göttingen, Germany.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
H.R. has served on the advisory boards of, given lectures for and received research grants from Abbott, Abbvie, Bayer Health Care, Boehringer Ingelheim, Brittania Pharmaceuticals, Cephalon, Desitin, GlaxoSmithKline, Lundbeck, Medtronic, MerckSerono, Novartis, Orion Pharma, Pfizer, Teva Pharmaceutical Industries, UCB, Valeant Pharmaceutical International, and Zambon. L.K declares no competing interests.
Rights and permissions
About this article
Cite this article
Klingelhoefer, L., Reichmann, H. Pathogenesis of Parkinson disease—the gut–brain axis and environmental factors. Nat Rev Neurol 11, 625–636 (2015). https://doi.org/10.1038/nrneurol.2015.197
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2015.197
This article is cited by
-
Amelioration of olfactory dysfunction in a mouse model of Parkinson’s disease via enhancing GABAergic signaling
Cell & Bioscience (2023)
-
Parkinson’s disease and gut microbiota: from clinical to mechanistic and therapeutic studies
Translational Neurodegeneration (2023)
-
Characterization of the partial volume effect along the axial field-of-view of the Biograph Vision Quadra total-body PET/CT system for multiple isotopes
EJNMMI Physics (2023)
-
Helicobacter hepaticus augmentation triggers Dopaminergic degeneration and motor disorders in mice with Parkinson’s disease
Molecular Psychiatry (2023)
-
Detection of SARS-CoV-2 viral proteins and genomic sequences in human brainstem nuclei
npj Parkinson's Disease (2023)