Key Points
-
Variants in the FUS gene are causative or risk factors for several neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS), frontotemporal lobar degeneration (FTLD), and essential tremor
-
Abnormal aggregation of the FUS protein has been reported in ALS, FTLD and polyglutamine diseases, suggesting an important role for this protein pathology in neurodegenerative diseases
-
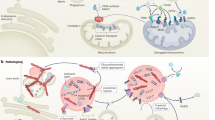
Under physiological conditions, FUS is mainly found in the nuclear compartment, where it is involved in the regulation of various cellular processes
-
In neurodegenerative diseases, FUS is primarily found in cytoplasmic inclusions that have variable morphology and distribution across distinct disease entities
-
Functional and animal studies have led to improved understanding of the pathogenetic mechanisms of FUS-related disorders; the results identify this protein as a promising therapeutic target
Abstract
The neurodegenerative diseases are a diverse group of disorders characterized by progressive loss of specific groups of neurons. These diseases affect different populations, and have a variable age of onset, clinical symptoms, and pathological findings. Variants in the FUS gene, which encodes an RNA-binding protein, have been identified as causative or risk factors for amyotrophic lateral sclerosis (ALS), essential tremor and rare forms of frontotemporal lobar degeneration (FTLD). Additionally, abnormal aggregation of FUS protein has been reported in multiple neurodegenerative diseases, including ALS, FTLD and the polyglutamine diseases, suggesting a role for FUS in the pathogenesis of these neurodegenerative diseases. This Review summarizes current understanding of the normal function of FUS, and describes its role in the pathology of ALS, FTLD, essential tremor and other neurodegenerative diseases. Comments on the underlying pathogenetic mechanisms of these FUS-related disorders are included. Finally, the clinical implications of recent advances in FUS research are discussed. Further understanding of the role of FUS in neurodegenerative diseases might lead to improvements in the treatment and prevention of these disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Soto, C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat. Rev. Neurosci. 4, 49–60 (2003).
Sleegers, K., Cruts, M. & Van Broeckhoven, C. Molecular pathways of frontotemporal lobar degeneration. Annu. Rev. Neurosci. 33, 71–88 (2010).
Neumann, M. et al. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain 132, 2922–2931 (2009).
Deng, H. X. et al. FUS-immunoreactive inclusions are a common feature in sporadic and non-SOD1 familial amyotrophic lateral sclerosis. Ann. Neurol. 67, 739–748 (2010).
Neumann, M. et al. Abundant FUS-immunoreactive pathology in neuronal intermediate filament inclusion disease. Acta Neuropathol. 118, 605–616 (2009).
Doi, H., Koyano, S., Suzuki, Y., Nukina, N. & Kuroiwa, Y. The RNA-binding protein FUS/TLS is a common aggregate-interacting protein in polyglutamine diseases. Neurosci. Res. 66, 131–133 (2010).
Woulfe, J., Gray, D. A. & Mackenzie, I. R. FUS-immunoreactive intranuclear inclusions in neurodegenerative disease. Brain Pathol. 20, 589–597 (2010).
Crozat, A., Aman, P., Mandahl, N. & Ron, D. Fusion of CHOP to a novel RNA-binding protein in human myxoid liposarcoma. Nature 363, 640–644 (1993).
Rabbitts, T. H., Forster, A., Larson, R. & Nathan, P. Fusion of the dominant negative transcription regulator CHOP with a novel gene FUS by translocation t(12;16) in malignant liposarcoma. Nat. Genet. 4, 175–180 (1993).
Tan, A. Y. & Manley, J. L. The TET family of proteins: functions and roles in disease. J. Mol. Cell Biol. 1, 82–92 (2009).
Lagier-Tourenne, C., Polymenidou, M. & Cleveland, D. W. TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration. Hum. Mol. Genet. 19, R46–R64 (2010).
Kwiatkowski, T. J. Jr et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323, 1205–1208 (2009).
Vance, C. et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323, 1208–1211 (2009).
Broustal, O. et al. FUS mutations in frontotemporal lobar degeneration with amyotrophic lateral sclerosis. J. Alzheimers Dis. 22, 765–769 (2010).
Van Langenhove, T. et al. Genetic contribution of FUS to frontotemporal lobar degeneration. Neurology 74, 366–371 (2010).
Merner, N. D. et al. Exome sequencing identifies FUS mutations as a cause of essential tremor. Am. J. Hum. Genet. 91, 313–319 (2012).
Zinszner, H., Sok, J., Immanuel, D., Yin, Y. & Ron, D. TLS (FUS) binds RNA in vivo and engages in nucleo-cytoplasmic shuttling. J. Cell Sci. 110, 1741–1750 (1997).
Iko, Y. et al. Domain architectures and characterization of an RNA-binding protein, TLS. J. Biol. Chem. 279, 44834–44840 (2004).
Dormann, D. et al. ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import. EMBO J. 29, 2841–2857 (2010).
Dormann, D. et al. Arginine methylation next to the PY-NLS modulates Transportin binding and nuclear import of FUS. EMBO J. 31, 4258–4275 (2012).
Baechtold, H. et al. Human 75-kDa DNA-pairing protein is identical to the pro-oncoprotein TLS/FUS and is able to promote D-loop formation. J. Biol. Chem. 274, 34337–34342 (1999).
Hicks, G. G. et al. Fus deficiency in mice results in defective B-lymphocyte development and activation, high levels of chromosomal instability and perinatal death. Nat. Genet. 24, 175–179 (2000).
Kuroda, M. et al. Male sterility and enhanced radiation sensitivity in TLS−/− mice. EMBO J. 19, 453–462 (2000).
Mastrocola, A. S., Kim, S. H., Trinh, A. T., Rodenkirch, L. A. & Tibbetts, R. S. The RNA binding protein fused in sarcoma (FUS) functions downstream of PARP in response to DNA damage. J. Biol. Chem. 288, 24731–24741 (2013).
Wang, W. Y. et al. Interaction of FUS and HDAC1 regulates DNA damage response and repair in neurons. Nat. Neurosci. 16, 1383–1391 (2013).
Rulten, S. L. et al. PARP-1 dependent recruitment of the amyotrophic lateral sclerosis-associated protein FUS/TLS to sites of oxidative DNA damage. Nucleic Acids Res. 42, 307–314 (2014).
Bertolotti, A., Lutz, Y., Heard, D. J., Chambon, P. & Tora, L. hTAF(II)68, a novel RNA/ssDNA-binding protein with homology to the pro-oncoproteins TLS/FUS and EWS is associated with both TFIID and RNA polymerase II. EMBO J. 15, 5022–5031 (1996).
Hallier, M., Lerga, A., Barnache, S., Tavitian, A. & Moreau-Gachelin, F. The transcription factor Spi-1/PU.1 interacts with the potential splicing factor TLS. J. Biol. Chem. 273, 4838–4842 (1998).
Uranishi, H. et al. Involvement of the pro-oncoprotein TLS (translocated in liposarcoma) in nuclear factor-κB p65-mediated transcription as a coactivator. J. Biol. Chem. 276, 13395–13401 (2001).
Wang, X. et al. Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature 454, 126–130 (2008).
Tan, A. Y. & Manley, J. L. TLS inhibits RNA polymerase III transcription. Mol. Cell. Biol. 30, 186–196 (2010).
Schwartz, J. C. et al. FUS binds the CTD of RNA polymerase II and regulates its phosphorylation at Ser2. Genes Dev. 26, 2690–2695 (2012).
Kwon, I. et al. Phosphorylation-regulated binding of RNA polymerase II to fibrous polymers of low-complexity domains. Cell 155, 1049–1060 (2013).
Tan, A. Y., Riley, T. R., Coady, T., Bussemaker, H. J. & Manley, J. L. TLS/FUS (translocated in liposarcoma/fused in sarcoma) regulates target gene transcription via single-stranded DNA response elements. Proc. Natl Acad. Sci. USA 109, 6030–6035 (2012).
Ishigaki, S. et al. Position-dependent FUS–RNA interactions regulate alternative splicing events and transcriptions. Sci. Rep. 2, 529 (2012).
Fujioka, Y. et al. FUS-regulated region- and cell-type-specific transcriptome is associated with cell selectivity in ALS/FTLD. Sci. Rep. 3, 2388 (2013).
Zinszner, H., Albalat, R. & Ron, D. A novel effector domain from the RNA-binding protein TLS or EWS is required for oncogenic transformation by CHOP. Genes Dev. 8, 2513–2526 (1994).
Zhang, W. J. & Wu, J. Y. Sip1, a novel RS domain-containing protein essential for pre-mRNA splicing. Mol. Cell. Biol. 18, 676–684 (1998).
Chansky, H. A., Hu, M., Hickstein, D. D. & Yang, L. Oncogenic TLS/ERG and EWS/Fli-1 fusion proteins inhibit RNA splicing mediated by YB-1 protein. Cancer Res. 61, 3586–3590 (2001).
Hartmuth, K. et al. Protein composition of human prespliceosomes isolated by a tobramycin affinity-selection method. Proc. Natl Acad. Sci. USA 99, 16719–16724 (2002).
Rappsilber, J., Ryder, U., Lamond, A. I. & Mann, M. Large-scale proteomic analysis of the human spliceosome. Genome Res. 12, 1231–1245 (2002).
Camats, M., Guil, S., Kokolo, M. & Bach-Elias, M. P68 RNA helicase (DDX5) alters activity of cis- and trans-acting factors of the alternative splicing of H-Ras. PLoS ONE 3, e2926 (2008).
Orozco, D. & Edbauer, D. FUS-mediated alternative splicing in the nervous system: consequences for ALS and FTLD. J. Mol. Med. (Berl.) 91, 1343–1354 (2013).
Orozco, D. et al. Loss of fused in sarcoma (FUS) promotes pathological Tau splicing. EMBO Rep. 13, 759–764 (2012).
Zhou, Y., Liu, S., Liu, G., Ozturk, A. & Hicks, G. G. ALS-associated FUS mutations result in compromised FUS alternative splicing and autoregulation. PLoS Genet. 9, e1003895 (2013).
Fujii, R. et al. The RNA binding protein TLS is translocated to dendritic spines by mGluR5 activation and regulates spine morphology. Curr. Biol. 15, 587–593 (2005).
Fujii, R. & Takumi, T. TLS facilitates transport of mRNA encoding an actin-stabilizing protein to dendritic spines. J. Cell Sci. 118, 5755–5765 (2005).
Gregory, R. I. et al. The Microprocessor complex mediates the genesis of microRNAs. Nature 432, 235–240 (2004).
Morlando, M. et al. FUS stimulates microRNA biogenesis by facilitating co-transcriptional Drosha recruitment. EMBO J. 31, 4502–4510 (2012).
Mackenzie, I. R., Rademakers, R. & Neumann, M. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol. 9, 995–1007 (2010).
Mitchell, J. D. & Borasio, G. D. Amyotrophic lateral sclerosis. Lancet 369, 2031–2041 (2007).
Chiò, A. et al. A two-stage genome-wide association study of sporadic amyotrophic lateral sclerosis. Hum. Mol. Genet. 18, 1524–1532 (2009).
Rosen, D. R. et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 (1993).
van Blitterswijk, M., DeJesus-Hernandez, M. & Rademakers, R. How do C9ORF72 repeat expansions cause amyotrophic lateral sclerosis and frontotemporal dementia: can we learn from other noncoding repeat expansion disorders? Curr. Opin. Neurol. 25, 689–700 (2012).
Bruijn, L. I. et al. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science 281, 1851–1854 (1998).
Belzil, V. V. et al. Mutations in FUS cause FALS and SALS in French and French Canadian populations. Neurology 73, 1176–1179 (2009).
Bäumer, D. et al. Juvenile ALS with basophilic inclusions is a FUS proteinopathy with FUS mutations. Neurology 75, 611–618 (2010).
Rademakers, R. et al. FUS gene mutations in familial and sporadic amyotrophic lateral sclerosis. Muscle Nerve 42, 170–176 (2010).
Mackenzie, I. R. et al. Pathological heterogeneity in amyotrophic lateral sclerosis with FUS mutations: two distinct patterns correlating with disease severity and mutation. Acta Neuropathol. 122, 87–98 (2011).
Mochizuki, Y. et al. Familial ALS with FUS P525L mutation: two Japanese sisters with multiple systems involvement. J. Neurol. Sci. 323, 85–92 (2012).
Suzuki, N. et al. FUS/TLS-immunoreactive neuronal and glial cell inclusions increase with disease duration in familial amyotrophic lateral sclerosis with an R521C FUS/TLS mutation. J. Neuropathol. Exp. Neurol. 71, 779–788 (2012).
Blair, I. P. et al. FUS mutations in amyotrophic lateral sclerosis: clinical, pathological, neurophysiological and genetic analysis. J. Neurol. Neurosurg. Psychiatry 81, 639–645 (2010).
Hewitt, C. et al. Novel FUS/TLS mutations and pathology in familial and sporadic amyotrophic lateral sclerosis. Arch. Neurol. 67, 455–461 (2010).
Suzuki, N. et al. FALS with FUS mutation in Japan, with early onset, rapid progress and basophilic inclusion. J. Hum. Genet. 55, 252–254 (2010).
Tateishi, T. et al. Multiple system degeneration with basophilic inclusions in Japanese ALS patients with FUS mutation. Acta Neuropathol. 119, 355–364 (2010).
Huang, E. J. et al. Extensive FUS-immunoreactive pathology in juvenile amyotrophic lateral sclerosis with basophilic inclusions. Brain Pathol. 20, 1069–1076 (2010).
Kobayashi, Z. et al. Occurrence of basophilic inclusions and FUS-immunoreactive neuronal and glial inclusions in a case of familial amyotrophic lateral sclerosis. J. Neurol. Sci. 293, 6–11 (2010).
Matsuoka, T. et al. An autopsied case of sporadic adult-onset amyotrophic lateral sclerosis with FUS-positive basophilic inclusions. Neuropathology 31, 71–76 (2011).
Groen, E. J. et al. FUS mutations in familial amyotrophic lateral sclerosis in the Netherlands. Arch. Neurol. 67, 224–230 (2010).
Troakes, C. et al. Transportin 1 colocalization with Fused in Sarcoma (FUS) inclusions is not characteristic for amyotrophic lateral sclerosis-FUS confirming disrupted nuclear import of mutant FUS and distinguishing it from frontotemporal lobar degeneration with FUS inclusions. Neuropathol. Appl. Neurobiol. 39, 553–561 (2013).
Oketa, Y., Higashida, K., Fukasawa, H., Tsukie, T. & Ono, S. Abundant FUS-immunoreactive pathology in the skin of sporadic amyotrophic lateral sclerosis. Acta Neurol. Scand. 128, 257–264 (2013).
Sieben, A. et al. The genetics and neuropathology of frontotemporal lobar degeneration. Acta Neuropathol. 124, 353–372 (2012).
McKhann, G. M. et al. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch. Neurol. 58, 1803–1809 (2001).
Forman, M. S. et al. Frontotemporal dementia: clinicopathological correlations. Ann. Neurol. 59, 952–962 (2006).
Arai, T. et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 351, 602–611 (2006).
Neumann, M. et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133 (2006).
Mackenzie, I. R. et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann. Neurol. 61, 427–434 (2007).
Dormann, D. & Haass, C. Fused in sarcoma (FUS): an oncogene goes awry in neurodegeneration. Mol. Cell. Neurosci. 56, 475–486 (2013).
Mackenzie, I. R., Foti, D., Woulfe, J. & Hurwitz, T. A. Atypical frontotemporal lobar degeneration with ubiquitin-positive, TDP-43-negative neuronal inclusions. Brain 131, 1282–1293 (2008).
Mackenzie, I. R. et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 119, 1–4 (2010).
Rademakers, R., Neumann, M. & Mackenzie, I. R. Advances in understanding the molecular basis of frontotemporal dementia. Nat. Rev. Neurol. 8, 423–434 (2012).
Kobayashi, Z. et al. Pathological features of FTLD-FUS in a Japanese population: analyses of nine cases. J. Neurol. Sci. 335, 89–95 (2013).
Cairns, N. J. et al. Clinical and neuropathologic variation in neuronal intermediate filament inclusion disease. Neurology 63, 1376–1384 (2004).
Yokota, O. et al. Basophilic inclusion body disease and neuronal intermediate filament inclusion disease: a comparative clinicopathological study. Acta Neuropathol. 115, 561–575 (2008).
Munoz, D. G. et al. FUS pathology in basophilic inclusion body disease. Acta Neuropathol. 118, 617–627 (2009).
Lee, E. B. et al. Topography of FUS pathology distinguishes late-onset BIBD from aFTLD-U. Acta Neuropathol. Commun. 1, 1–11 (2013).
Mackenzie, I. R. et al. Distinct pathological subtypes of FTLD-FUS. Acta Neuropathol. 121, 207–218 (2011).
Chen-Plotkin, A. S., Lee, V. M. & Trojanowski, J. Q. TAR DNA-binding protein 43 in neurodegenerative disease. Nat. Rev. Neurol. 6, 211–220 (2010).
Doi, H. et al. RNA-binding protein TLS is a major nuclear aggregate-interacting protein in huntingtin exon 1 with expanded polyglutamine-expressing cells. J. Biol. Chem. 283, 6489–6500 (2008).
Chiò, A. et al. Two Italian kindreds with familial amyotrophic lateral sclerosis due to FUS mutation. Neurobiol. Aging 30, 1272–1275 (2009).
Ticozzi, N. et al. Analysis of FUS gene mutation in familial amyotrophic lateral sclerosis within an Italian cohort. Neurology 73, 1180–1185 (2009).
Bosco, D. A. et al. Mutant FUS proteins that cause amyotrophic lateral sclerosis incorporate into stress granules. Hum. Mol. Genet. 19, 4160–4175 (2010).
Corrado, L. et al. Mutations of FUS gene in sporadic amyotrophic lateral sclerosis. J. Med. Genet. 47, 190–194 (2010).
Damme, P. V. et al. The occurrence of mutations in FUS in a Belgian cohort of patients with familial ALS. Eur. J. Neurol. 17, 754–756 (2010).
DeJesus-Hernandez, M. et al. De novo truncating FUS gene mutation as a cause of sporadic amyotrophic lateral sclerosis. Hum. Mutat. 31, E1377–E1389 (2010).
Millecamps, S. et al. SOD1, ANG, VAPB, TARDBP, and FUS mutations in familial amyotrophic lateral sclerosis: genotype-phenotype correlations. J. Med. Genet. 47, 554–560 (2010).
Waibel, S., Neumann, M., Rabe, M., Meyer, T. & Ludolph, A. C. Novel missense and truncating mutations in FUS/TLS in familial ALS. Neurology 75, 815–817 (2010).
Yamamoto-Watanabe, Y. et al. A Japanese ALS6 family with mutation R521C in the FUS/TLS gene: a clinical, pathological and genetic report. J. Neurol. Sci. 296, 59–63 (2010).
Yan, J. et al. Frameshift and novel mutations in FUS in familial amyotrophic lateral sclerosis and ALS/dementia. Neurology 75, 807–814 (2010).
Belzil, V. V. et al. Identification of novel FUS mutations in sporadic cases of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 12, 113–117 (2011).
Chiò, A. et al. A de novo missense mutation of the FUS gene in a “true” sporadic ALS case. Neurobiol. Aging 32, 553.e23–553.e26 (2011).
Drepper, C., Herrmann, T., Wessig, C., Beck, M. & Sendtner, M. C-terminal FUS/TLS mutations in familial and sporadic ALS in Germany. Neurobiol. Aging 32, 548.e1–548.e4 (2011).
Fecto, F. & Siddique, T. Making connections: pathology and genetics link amyotrophic lateral sclerosis with frontotemporal lobe dementia. J. Mol. Neurosci. 45, 663–675 (2011).
Lai, S. L. et al. FUS mutations in sporadic amyotrophic lateral sclerosis. Neurobiol. Aging 32, 550.e1–550.e4 (2011).
Robertson, J. et al. A novel double mutation in FUS gene causing sporadic ALS. Neurobiol. Aging 32, 553.e27–553.e30 (2011).
Syriani, E., Morales, M. & Gamez, J. FUS/TLS gene mutations are the second most frequent cause of familial ALS in the Spanish population. Amyotroph. Lateral Scler. 12, 118–123 (2011).
Tsai, C. P. et al. FUS, TARDBP, and SOD1 mutations in a Taiwanese cohort with familial ALS. Neurobiol. Aging 32, 553.e13–553.e21 (2011).
Belzil, V. V. et al. Novel FUS deletion in a patient with juvenile amyotrophic lateral sclerosis. Arch. Neurol. 69, 653–656 (2012).
Brown, J. A. et al. SOD1, ANG, TARDBP and FUS mutations in amyotrophic lateral sclerosis: a United States clinical testing lab experience. Amyotroph. Lateral Scler. 13, 217–222 (2012).
Chiò, A. et al. Extensive genetics of ALS: a population-based study in Italy. Neurology 79, 1983–1989 (2012).
Hara, M. et al. Lower motor neuron disease caused by a novel FUS/TLS gene frameshift mutation. J. Neurol. 259, 2237–2239 (2012).
Kwon, M. J. et al. Screening of the SOD1, FUS, TARDBP, ANG, and OPTN mutations in Korean patients with familial and sporadic ALS. Neurobiol. Aging 33, 1017.e17–1017.e23 (2012).
Lattante, S. et al. Contribution of major amyotrophic lateral sclerosis genes to the etiology of sporadic disease. Neurology 79, 66–72 (2012).
Nagayama, S. et al. Novel FUS mutation in patients with sporadic amyotrophic lateral sclerosis and corticobasal degeneration. J. Clin. Neurosci. 19, 1738–1739 (2012).
Sproviero, W. et al. FUS mutations in sporadic amyotrophic lateral sclerosis: clinical and genetic analysis. Neurobiol. Aging 33, 837.e1–837.e5 (2012).
van Blitterswijk, M. et al. Evidence for an oligogenic basis of amyotrophic lateral sclerosis. Hum. Mol. Genet. 21, 3776–3784 (2012).
Yamashita, S. et al. Sporadic juvenile amyotrophic lateral sclerosis caused by mutant FUS/TLS: possible association of mental retardation with this mutation. J. Neurol. 259, 1039–1044 (2012).
Zou, Z. Y. et al. Screening of the FUS gene in familial and sporadic amyotrophic lateral sclerosis patients of Chinese origin. Eur. J. Neurol. 19, 977–983 (2012).
Zou, Z. Y. et al. De novo FUS gene mutations are associated with juvenile-onset sporadic amyotrophic lateral sclerosis in China. Neurobiol. Aging 34, 1312.e1–1312.e8 (2013).
Sabatelli, M. et al. Mutations in the 3′ untranslated region of FUS causing FUS overexpression are associated with amyotrophic lateral sclerosis. Hum. Mol. Genet. 22, 4748–4755 (2013).
Dormann, D. & Haass, C. TDP-43 and FUS: a nuclear affair. Trends Neurosci. 34, 339–348 (2011).
Gal, J. et al. Nuclear localization sequence of FUS and induction of stress granules by ALS mutants. Neurobiol. Aging 32, 2323.e27–2323.e40 (2011).
Ito, D., Seki, M., Tsunoda, Y., Uchiyama, H. & Suzuki, N. Nuclear transport impairment of amyotrophic lateral sclerosis-linked mutations in FUS/TLS. Ann. Neurol. 69, 152–162 (2011).
Kino, Y. et al. Intracellular localization and splicing regulation of FUS/TLS are variably affected by amyotrophic lateral sclerosis-linked mutations. Nucleic Acids Res. 39, 2781–2798 (2011).
Niu, C. et al. FUS-NLS/Transportin 1 complex structure provides insights into the nuclear targeting mechanism of FUS and the implications in ALS. PLoS ONE 7, e47056 (2012).
Zhang, Z. C. & Chook, Y. M. Structural and energetic basis of ALS-causing mutations in the atypical proline-tyrosine nuclear localization signal of the fused in sarcoma protein (FUS). Proc. Natl Acad. Sci. USA 109, 12017–12021 (2012).
Nomura, T. et al. Intranuclear aggregation of mutant FUS/TLS as a molecular pathomechanism of amyotrophic lateral sclerosis. J. Biol. Chem. 289, 1192–1202 (2014).
Huey, E. D. et al. FUS and TDP43 genetic variability in FTD and CBS. Neurobiol. Aging 33, 1016.e9–1016.e17 (2012).
Deng, H., Le, W. & Jankovic, J. Genetics of essential tremor. Brain 130, 1456–1464 (2007).
Bermejo-Pareja, F. Essential tremor—a neurodegenerative disorder associated with cognitive defects? Nat. Rev. Neurol. 7, 273–282 (2011).
Rajput, A. et al. Identification of FUS p.R377W in essential tremor. Eur. J. Neurol. 21, 361–363 (2014).
Wu, Y. R. et al. Identification of a novel risk variant in the FUS gene in essential tremor. Neurology 81, 541–544 (2013).
Zheng, W. et al. Genetic analysis of the fused in sarcoma gene in Chinese Han patients with essential tremor. Neurobiol. Aging 34, 2078.e3–2078.e4 (2013).
Hedera, P., Davis, T. L., Phibbs, F. T., Charles, P. D. & Ledoux, M. S. FUS in familial essential tremor—the search for common causes is still on. Parkinsonism Relat. Disord. 19, 818–820 (2013).
Labbe, C. et al. Investigating the role of FUS exonic variants in essential tremor. Parkinsonism Relat. Disord. 19, 755–757 (2013).
Ortega-Cubero, S. et al. Fused in Sarcoma (FUS) gene mutations are not a frequent cause of essential tremor in Europeans. Neurobiol. Aging 34, 2441.e9–2441.e11 (2013).
Parmalee, N. et al. Genetic analysis of the FUS/TLS gene in essential tremor. Eur. J. Neurol. 20, 534–539 (2013).
Gao, K. et al. Genetic analysis of the fused in sarcoma gene in Chinese Han patients with Parkinson's disease. Parkinsonism Relat. Disord. 20, 119–121 (2014).
Fekete, R. & Jankovic, J. Revisiting the relationship between essential tremor and Parkinson's disease. Mov. Disord. 26, 391–398 (2011).
Stamper, C. et al. Neuronal gene expression correlates of Parkinson's disease with dementia. Mov. Disord. 23, 1588–1595 (2008).
Lee, E. B., Lee, V. M. & Trojanowski, J. Q. Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration. Nat. Rev. Neurosci. 13, 38–50 (2012).
Ling, S. C. et al. ALS-associated mutations in TDP-43 increase its stability and promote TDP-43 complexes with FUS/TLS. Proc. Natl Acad. Sci. USA 107, 13318–13323 (2010).
Kabashi, E. et al. FUS and TARDBP but not SOD1 interact in genetic models of amyotrophic lateral sclerosis. PLoS Genet. 7, e1002214 (2011).
Kryndushkin, D., Wickner, R. B. & Shewmaker, F. FUS/TLS forms cytoplasmic aggregates, inhibits cell growth and interacts with TDP-43 in a yeast model of amyotrophic lateral sclerosis. Protein Cell 2, 223–236 (2011).
Lanson, N. A., Jr et al. A Drosophila model of FUS-related neurodegeneration reveals genetic interaction between FUS and TDP-43. Hum. Mol. Genet. 20, 2510–2523 (2011).
Wang, J. W., Brent, J. R., Tomlinson, A., Shneider, N. A. & McCabe, B. D. The ALS-associated proteins FUS and TDP-43 function together to affect Drosophila locomotion and life span. J. Clin. Invest. 121, 4118–4126 (2011).
Neumann, M. et al. FET proteins TAF15 and EWS are selective markers that distinguish FTLD with FUS pathology from amyotrophic lateral sclerosis with FUS mutations. Brain 134, 2595–2609 (2011).
Mackenzie, I. R. & Neumann, M. FET proteins in frontotemporal dementia and amyotrophic lateral sclerosis. Brain Res. 1462, 40–43 (2012).
Thomsen, C., Grundevik, P., Elias, P., Stahlberg, A. & Aman, P. A conserved N-terminal motif is required for complex formation between FUS, EWSR1, TAF15 and their oncogenic fusion proteins. FASEB J. 27, 4965–4974 (2013).
Couthouis, J. et al. A yeast functional screen predicts new candidate ALS disease genes. Proc. Natl Acad. Sci. USA 108, 20881–20890 (2011).
Couthouis, J. et al. Evaluating the role of the FUS/TLS-related gene EWSR1 in amyotrophic lateral sclerosis. Hum. Mol. Genet. 21, 2899–2911 (2012).
Ticozzi, N. et al. Mutational analysis reveals the FUS homolog TAF15 as a candidate gene for familial amyotrophic lateral sclerosis. Am. J. Med. Genet. B Neuropsychiatr. Genet. 156, 285–290 (2011).
Sen, A. et al. Genetic circuitry of Survival motor neuron, the gene underlying spinal muscular atrophy. Proc. Natl Acad. Sci. USA 110, E2371–E2380 (2013).
Yamazaki, T. et al. FUS–SMN protein interactions link the motor neuron diseases ALS and SMA. Cell Rep. 2, 799–806 (2012).
Groen, E. J. et al. ALS-associated mutations in FUS disrupt the axonal distribution and function of SMN. Hum. Mol. Genet. 22, 3690–3704 (2013).
Blokhuis, A. M., Groen, E. J., Koppers, M., van den Berg, L. H. & Pasterkamp, R. J. Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol. 125, 777–794 (2013).
Ling, S. C., Polymenidou, M. & Cleveland, D. W. Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron 79, 416–438 (2013).
Bentmann, E. et al. Requirements for stress granule recruitment of fused in sarcoma (FUS) and TAR DNA-binding protein of 43 kDa (TDP-43). J. Biol. Chem. 287, 23079–23094 (2012).
Kato, M. et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 149, 753–767 (2012).
Daigle, J. G. et al. RNA-binding ability of FUS regulates neurodegeneration, cytoplasmic mislocalization and incorporation into stress granules associated with FUS carrying ALS-linked mutations. Hum. Mol. Genet. 22, 1193–1205 (2013).
Vance, C. et al. ALS mutant FUS disrupts nuclear localization and sequesters wild-type FUS within cytoplasmic stress granules. Hum. Mol. Genet. 22, 2676–2688 (2013).
Andersson, M. K. et al. The multifunctional FUS, EWS and TAF15 proto-oncoproteins show cell type-specific expression patterns and involvement in cell spreading and stress response. BMC Cell Biol. 9, 37 (2008).
Blechingberg, J., Luo, Y., Bolund, L., Damgaard, C. K. & Nielsen, A. L. Gene expression responses to FUS, EWS, and TAF15 reduction and stress granule sequestration analyses identifies FET-protein non-redundant functions. PLoS ONE 7, e46251 (2012).
Bentmann, E., Haass, C. & Dormann, D. Stress granules in neurodegeneration—lessons learnt from TAR DNA binding protein of 43 kDa and fused in sarcoma. FEBS J. 280, 4348–4370 (2013).
Aulas, A., Stabile, S. & Vande Velde, C. Endogenous TDP-43, but not FUS, contributes to stress granule assembly via G3BP. Mol. Neurodegener. 7, 54 (2012).
Li, Y. R., King, O. D., Shorter, J. & Gitler, A. D. Stress granules as crucibles of ALS pathogenesis. J. Cell Biol. 201, 361–372 (2013).
Fushimi, K. et al. Expression of human FUS/TLS in yeast leads to protein aggregation and cytotoxicity, recapitulating key features of FUS proteinopathy. Protein Cell 2, 141–149 (2011).
Baron, D. M. et al. Amyotrophic lateral sclerosis-linked FUS/TLS alters stress granule assembly and dynamics. Mol. Neurodegener. 8, 30 (2013).
Shelkovnikova, T. A., Robinson, H., Connor-Robson, N. & Buchman, V. L. Recruitment into stress granules prevents irreversible aggregation of FUS protein mislocalized to the cytoplasm. Cell Cycle 12, 3194–3202 (2013).
Cushman, M., Johnson, B. S., King, O. D., Gitler, A. D. & Shorter, J. Prion-like disorders: blurring the divide between transmissibility and infectivity. J. Cell Sci. 123, 1191–1201 (2010).
King, O. D., Gitler, A. D. & Shorter, J. The tip of the iceberg: RNA-binding proteins with prion-like domains in neurodegenerative disease. Brain Res. 1462, 61–80 (2012).
Gitler, A. D. & Shorter, J. RNA-binding proteins with prion-like domains in ALS and FTLD-U. Prion 5, 179–187 (2011).
Polymenidou, M. & Cleveland, D. W. The seeds of neurodegeneration: prion-like spreading in ALS. Cell 147, 498–508 (2011).
Sun, Z. et al. Molecular determinants and genetic modifiers of aggregation and toxicity for the ALS disease protein FUS/TLS. PLoS Biol. 9, e1000614 (2011).
Ju, S. et al. A yeast model of FUS/TLS-dependent cytotoxicity. PLoS Biol. 9, e1001052 (2011).
Han, T. W. et al. Cell-free formation of RNA granules: bound RNAs identify features and components of cellular assemblies. Cell 149, 768–779 (2012).
Schwartz, J. C., Wang, X., Podell, E. R. & Cech, T. R. RNA seeds higher-order assembly of FUS protein. Cell Rep. 5, 918–925 (2013).
Frost, B. & Diamond, M. I. Prion-like mechanisms in neurodegenerative diseases. Nat. Rev. Neurosci. 11, 155–159 (2010).
Hoell, J. I. et al. RNA targets of wild-type and mutant FET family proteins. Nat. Struct. Mol. Biol. 18, 1428–1431 (2011).
Lagier-Tourenne, C. et al. Divergent roles of ALS-linked proteins FUS/TLS and TDP-43 intersect in processing long pre-mRNAs. Nat. Neurosci. 15, 1488–1497 (2012).
Rogelj, B. et al. Widespread binding of FUS along nascent RNA regulates alternative splicing in the brain. Sci. Rep. 2, 603 (2012).
Tradewell, M. L. et al. Arginine methylation by PRMT1 regulates nuclear-cytoplasmic localization and toxicity of FUS/TLS harbouring ALS-linked mutations. Hum. Mol. Genet. 21, 136–149 (2012).
Scaramuzzino, C. et al. Protein arginine methyltransferase 1 and 8 interact with FUS to modify its sub-cellular distribution and toxicity in vitro and in vivo. PLoS ONE 8, e61576 (2013).
Yamaguchi, A. & Kitajo, K. The effect of PRMT1-mediated arginine methylation on the subcellular localization, stress granules, and detergent-insoluble aggregates of FUS/TLS. PLoS ONE 7, e49267 (2012).
Sama, R. R. et al. FUS/TLS assembles into stress granules and is a prosurvival factor during hyperosmolar stress. J. Cell. Physiol. 228, 2222–2231 (2013).
Xia, R. et al. Motor neuron apoptosis and neuromuscular junction perturbation are prominent features in a Drosophila model of Fus-mediated ALS. Mol. Neurodegener. 7, 10 (2012).
Sasayama, H. et al. Knockdown of the Drosophila fused in sarcoma (FUS) homologue causes deficient locomotive behavior and shortening of motoneuron terminal branches. PLoS ONE 7, e39483 (2012).
Armstrong, G. A. & Drapeau, P. Loss and gain of FUS function impair neuromuscular synaptic transmission in a genetic model of ALS. Hum. Mol. Genet. 22, 4282–4292 (2013).
Chen, Y. et al. Expression of human FUS protein in Drosophila leads to progressive neurodegeneration. Protein Cell 2, 477–486 (2011).
Mitchell, J. C. et al. Overexpression of human wild-type FUS causes progressive motor neuron degeneration in an age- and dose-dependent fashion. Acta Neuropathol. 125, 273–288 (2013).
Shelkovnikova, T. A. et al. Fused in sarcoma (FUS) protein lacking nuclear localization signal (NLS) and major RNA binding motifs triggers proteinopathy and severe motor phenotype in transgenic mice. J. Biol. Chem. 288, 25266–25274 (2013).
Murakami, T. et al. ALS mutations in FUS cause neuronal dysfunction and death in Caenorhabditis elegans by a dominant gain-of-function mechanism. Hum. Mol. Genet. 21, 1–9 (2012).
Vaccaro, A. et al. Methylene blue protects against TDP-43 and FUS neuronal toxicity in C. elegans and D. rerio. PLoS ONE 7, e42117 (2012).
Huang, C. et al. FUS transgenic rats develop the phenotypes of amyotrophic lateral sclerosis and frontotemporal lobar degeneration. PLoS Genet. 7, e1002011 (2011).
Huang, C. et al. Entorhinal cortical neurons are the primary targets of FUS mislocalization and ubiquitin aggregation in FUS transgenic rats. Hum. Mol. Genet. 21, 4602–4614 (2012).
Vaccaro, A. et al. Mutant TDP-43 and FUS cause age-dependent paralysis and neurodegeneration in C. elegans. PLoS ONE 7, e31321 (2012).
Verbeeck, C. et al. Expression of Fused in sarcoma mutations in mice recapitulates the neuropathology of FUS proteinopathies and provides insight into disease pathogenesis. Mol. Neurodegener. 7, 53 (2012).
Andersen, P. M. & Al-Chalabi, A. Clinical genetics of amyotrophic lateral sclerosis: what do we really know? Nat. Rev. Neurol. 7, 603–615 (2011).
Urwin, H. et al. FUS pathology defines the majority of tau- and TDP-43-negative frontotemporal lobar degeneration. Acta Neuropathol. 120, 33–41 (2010).
McClellan, J. & King, M. C. Genetic heterogeneity in human disease. Cell 141, 210–217 (2010).
Deng, H., Gao, K. & Jankovic, J. The genetics of Tourette syndrome. Nat. Rev. Neurol. 8, 203–213 (2012).
Cookson, M. R. The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson's disease. Nat. Rev. Neurosci. 11, 791–797 (2010).
Da Cruz, S. & Cleveland, D. W. Understanding the role of TDP-43 and FUS/TLS in ALS and beyond. Curr. Opin. Neurobiol. 21, 904–919 (2011).
Lanson, N. A. Jr & Pandey, U. B. FUS-related proteinopathies: lessons from animal models. Brain Res. 1462, 44–60 (2012).
Acknowledgements
H.D.'s research was funded by National Natural Science Foundation of China (81271921, 81101339), the Sheng Hua Scholars Program of Central South University, the Fundamental Research Funds for the Central Universities (2011JQ014), Research Fund for the Doctoral Program of Higher Education of China (20110162110,026), and the Construction Fund for Key Subjects of the Third Xiangya Hospital, Central South University. K.G.'s research was supported by the Fundamental Research Funds for the Central Universities of Central South University (2012zzts120). J.J. receives support from the National Parkinson Foundation.
Author information
Authors and Affiliations
Contributions
H.D. and K.G. contributed equally to researching data for the article, discussions of the content, and writing the article. J.J. contributed to review and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
Summary of clinical and genetic features of ALS patients with FUS mutations (DOC 154 kb)
Rights and permissions
About this article
Cite this article
Deng, H., Gao, K. & Jankovic, J. The role of FUS gene variants in neurodegenerative diseases. Nat Rev Neurol 10, 337–348 (2014). https://doi.org/10.1038/nrneurol.2014.78
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2014.78
This article is cited by
-
Nuclear-import receptors as gatekeepers of pathological phase transitions in ALS/FTD
Molecular Neurodegeneration (2024)
-
Cerebrospinal fluid and blood exosomes as biomarkers for amyotrophic lateral sclerosis; a systematic review
Diagnostic Pathology (2024)
-
A chaperone-like function of FUS ensures TAZ condensate dynamics and transcriptional activation
Nature Cell Biology (2024)
-
Disruption of MAM integrity in mutant FUS oligodendroglial progenitors from hiPSCs
Acta Neuropathologica (2024)
-
Digital color-coded molecular barcoding reveals dysregulation of common FUS and FMRP targets in soma and neurites of ALS mutant motoneurons
Cell Death Discovery (2023)