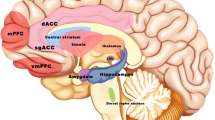
Key Points
-
Chronic pain is common and has substantial negative consequences for individuals and society
-
As the brain is ultimately the organ that processes pain information, treatments that target brain activity have the potential to provide pain relief
-
Solid evidence indicates that hypnosis has short-term and long-term benefits for a variety of pain problems, and should be considered as a first-line treatment given its demonstrated efficacy and positive side-effect profile
-
Training in meditation shows promise for reducing chronic pain, although more research is needed to confirm the initial findings
-
Noninvasive brain stimulation is potentially effective for reducing chronic pain in the short term, but preliminary evidence suggests that brain stimulation alone might not have long-term benefits
-
Neurofeedback has some potential for reducing chronic pain, although the research findings suggest weak effects when this technique is used alone
Abstract
Chronic pain is common, and the available treatments do not provide adequate relief for most patients. Neuromodulatory interventions that modify brain processes underlying the experience of pain have the potential to provide substantial relief for some of these patients. The purpose of this Review is to summarize the state of knowledge regarding the efficacy and mechanisms of noninvasive neuromodulatory treatments for chronic pain. The findings provide support for the efficacy and positive side-effect profile of hypnosis, and limited evidence for the potential efficacy of meditation training, noninvasive electrical stimulation procedures, and neurofeedback procedures. Mechanisms research indicates that hypnosis influences multiple neurophysiological processes involved in the experience of pain. Evidence also indicates that mindfulness meditation has both immediate and long-term effects on cortical structures and activity involved in attention, emotional responding and pain. Less is known about the mechanisms of other neuromodulatory treatments. On the basis of the data discussed in this Review, training in the use of self-hypnosis might be considered a viable 'first-line' approach to treat chronic pain. More-definitive research regarding the benefits and costs of meditation training, noninvasive brain stimulation and neurofeedback is needed before these treatments can be recommended for the treatment of chronic pain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Breivik, H., Collett, B., Ventafridda, V., Cohen, R. & Gallacher, D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur. J. Pain 10, 287–333 (2006).
Smith, B. H. & Torrance, N. Epidemiology of neuropathic pain and its impact on quality of life. Curr. Pain Headache Rep. 16, 191–198 (2012).
Toth, C., Lander, J. & Wiebe, S. The prevalence and impact of chronic pain with neuropathic pain symptoms in the general population. Pain Med. 10, 918–929 (2009).
Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research (The National Academics Press, 2011).
Huguet, A. & Miró, J. The severity of chronic pediatric pain: an epidemiological study. J. Pain 9, 226–236 (2008).
Tsang, A. et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression–anxiety disorders. J. Pain 9, 883–891 (2008).
Langley, P. C., Van Litsenburg, C., Cappelleri, J. C. & Carroll, D. The burden associated with neuropathic pain in Western Europe. J. Med. Econ. 16, 85–95 (2013).
Vranken, J. H. Elucidation of pathophysiology and treatment of neuropathic pain. Cent. Nerv. Syst. Agents Med. Chem. 12, 304–314 (2012).
Jefferies, K. Treatment of neuropathic pain. Semin. Neurol. 30, 425–432 (2010).
Baron, R., Binder, A. & Wasner, G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 9, 807–819 (2010).
Furlan, A. D., Sandoval, J. A., Mailis-Gagnon, A. & Tunks, E. Opioids for chronic noncancer pain: a meta-analysis of effectiveness and side effects. CMAJ 174, 1589–1594 (2006).
Apkarian, A. V., Hashmi, J. A. & Baliki, M. N. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain 152 (Suppl. 3), S49–S64 (2011).
Jensen, M., Hakimian, S., Sherlin, L. H. & Fregni, F. New insights into neuromodulatory approaches for the treatment of pain. J. Pain 9, 193–199 (2008).
Kihlstrom, J. F. Hypnosis. Annu. Rev. Psychol. 36, 385–418 (1985).
Oakley, D. A. & Halligan, P. W. Hypnotic suggestion: opportunities for cognitive neuroscience. Nat. Rev. Neurosci. 14, 565–576 (2013).
Derbyshire, S. W., Whalley, M. G. & Oakley, D. A. Fibromyalgia pain and its modulation by hypnotic and non-hypnotic suggestion: an fMRI analysis. Eur. J. Pain 13, 542–550 (2009).
Derbyshire, S. W., Whalley, M. G., Stenger, V. A. & Oakley, D. A. Cerebral activation during hypnotically induced and imagined pain. Neuroimage 23, 392–401 (2004).
Jensen, M. P. Hypnosis for Chronic Pain Management: Therapist Guide (Oxford University Press, 2011).
Jensen, M. & Patterson, D. R. Hypnotic treatment of chronic pain. J. Behav. Med. 29, 95–124 (2006).
Patterson, D. R. & Jensen, M. P. Hypnosis and clinical pain. Psychol. Bull. 29, 495–521 (2003).
Tome-Pires, C. & Miró, J. Hypnosis for the management of chronic and cancer procedure-related pain in children. Int. J. Clin. Exp. Hypn. 60, 432–457 (2012).
Montgomery, G. H., DuHamel, K. N. & Redd, W. H. A meta-analysis of hypnotically induced analgesia: how effective is hypnosis? Int. J. Clin. Exp. Hypn. 48, 138–153 (2000).
Dworkin, R. H. et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain 146, 238–244 (2009).
Jensen, M. P. et al. A comparison of self-hypnosis versus progressive muscle relaxation in patients with multiple sclerosis and chronic pain. Int. J. Clin. Exp. Hypn. 57, 198–221 (2009).
Jensen, M. P. et al. Hypnotic analgesia for chronic pain in persons with disabilities: a case series. Int. J. Clin. Exp. Hypn. 53, 198–228 (2005).
Jensen, M. P. et al. Effects of self-hypnosis training and EMG biofeedback relaxation training on chronic pain in persons with spinal-cord injury. Int. J. Clin. Exp. Hypn. 57, 239–268 (2009).
Dworkin, R. H. et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J. Pain 9, 105–121 (2008).
Hilgard, E. & Hilgard, J. Hypnosis in the Relief of Pain 2nd edn (William Kaufmam, 1975).
Crawford, H. J., Kitner-Triolo, M., Clarke, S. W. & Olesko, B. Transient positive and negative experiences accompanying stage hypnosis. J. Abnorm. Psychol. 101, 663–667 (1992).
Hammond, D. C. Review of the efficacy of clinical hypnosis with headaches and migraines. Int. J. Clin. Exp. Hypn. 55, 207–219 (2007).
Crasilneck, H. B. Hypnosis in the control of chronic low back pain. Am. J. Clin. Hypn. 22, 71–78 (1979).
Crawford, H. J. et al. Hypnotic analgesia: 1. Somatosensory event-related potential changes to noxious stimuli and 2. Transfer learning to reduce chronic low back pain. Int. J. Clin. Exp. Hypn. 46, 93–132 (1998).
Jack, M. A. The use of hypnosis for a patient with chronic pain. Contemp. Hypn. 16, 231–237 (1999).
Melzack, R. & Perry, C. Self-regulation of pain: the use of alpha-feedback and hypnotic training for the control of chronic pain. Exp. Neurol. 46, 452–469 (1975).
Sachs, L. B., Feuerstein, M. & Vitale, J. H. Hypnotic self-regulation of chronic pain. Am. J. Clin. Hypn. 20, 106–113 (1977).
Mott, T. Jr. Untoward effects associated with hypnosis. Psychiatr. Med. 10, 119–128 (1992).
Stewart, J. H. Hypnosis in contemporary medicine. Mayo Clin. Proc. 80, 511–524 (2005).
Jensen, M. P. et al. Satisfaction with, and the beneficial side effects of, hypnotic analgesia. Int. J. Clin. Exp. Hypn. 54, 432–447 (2006).
De Benedittis, G. Understanding the multidimensional mechanisms of hypnotic analgesia. Contemp. Hypn. 20, 59–80 (2003).
Jensen, M. P. The neurophysiology of pain perception and hypnotic analgesia: implications for clinical practice. Am. J. Clin. Hypn. 51, 123–148 (2008).
Rainville, P., Duncan, G. H., Price, D. D., Carrier, B. & Bushnell, M. C. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277, 968–971 (1997).
Hofbauer, R. K., Rainville, P., Duncan, G. H. & Bushnell, M. C. Cortical representation of the sensory dimension of pain. J. Neurophysiol. 86, 402–411 (2001).
Oakley, D. A., Deeley, Q. & Halligan, P. W. Hypnotic depth and response to suggestion under standardized conditions and during fMRI scanning. Int. J. Clin. Exp. Hypn. 55, 32–58 (2007).
Gruzelier, J. H. Frontal functions, connectivity and neural efficiency underpinning hypnosis and hypnotic susceptibility. Contemp. Hypn. 23, 15–32 (2006).
Chapman, L. F., Goodell, H. & Wolff, H. G. Changes in tissue vulnerability induced during hypnotic suggestion. J. Psychosom. Res. 4, 99–105 (1959).
Hammond, D. C., Keye, W. R. & Grant, C. W. Jr. Hypnotic analgesia with burns: an initial study. Am. J. Clin. Hypn. 26, 56–59 (1983).
Kiernan, B. D., Dane, J. R., Phillips, L. H. & Price, D. D. Hypnotic analgesia reduces R-III nociceptive reflex: further evidence concerning the multifactorial nature of hypnotic analgesia. Pain 60, 39–47 (1995).
Danziger, N. et al. Different strategies of modulation can be operative during hypnotic analgesia: a neurophysiological study. Pain 75, 85–92 (1998).
Crawford, H. J., Knebel, T. & Vendemia, J. The nature of hypnotic analgesia: neurophysiological foundation and evidence. Contemp. Hypn. 15, 22–33 (1998).
Fingelkurts, A. A., Kallio, S. & Revonsuo, A. Cortex functional connectivity as a neurophysiological correlate of hypnosis: an EEG case study. Neuropsychologia 45, 1452–1462 (2007).
Faymonville, M. E. et al. Increased cerebral functional connectivity underlying the antinociceptive effects of hypnosis. Brain Res. Cogn. Brain Res. 17, 255–262 (2003).
Wagstaff, G. F., David, D., Kirsch, I. & Lynn, S. J. in Handbook of Clinical Hypnosis (eds Lynn, S. J. et al.) 179–208 (American Psychological Association, 2010).
Sadler, P. & Woody, E. in Handbook of Clinical Hypnosis (eds Lynn, S. J. et al.) 151–178 (American Psychological Association, 2010).
Crawford, H. J. & Gruzelier, J. H. in Contemporary Hypnosis Research (eds Fromm, E. & Nash, M. R.) 227–266 (Guilford Press, 1992).
Gruzelier, J. A theory of alpha/theta neurofeedback, creative performance enhancement, long distance functional connectivity and psychological integration. Cogn. Process. 10 (Suppl. 1), S101–S109 (2009).
Lutz, A., Slagter, H. A., Dunne, J. D. & Davidson, R. J. Attention regulation and monitoring in meditation. Trends Cogn. Sci. 12, 163–169 (2008).
Grant, J. A. Meditative analgesia: the current state of the field. Ann. N. Y. Acad. Sci. http://dx.doi.org/10.1111/nyas.12282.
Kabat-Zinn, J. Mindfulness-based interventions in context: past, present, and future. Clin. Psychol. (New York) 10, 144–156 (2003).
Shapiro, S. L. & Carlson, L. E. The Art and Science of Mindfulness: Integrating Mindfulness into Psychology and the Helping Professions (American Psychological Association, 2009).
Fjorback, L. O., Arendt, M., Ornbøl, E., Fink, P. & Walach, H. Mindfulness-based stress reduction and mindfulness-based cognitive therapy: a systematic review of randomized controlled trials. Acta Psychiatr. Scand. 124, 102–119 (2011).
Grossman, P., Niemann, L., Schmidt, S. & Walach, H. Mindfulness-based stress reduction and health benefits. A meta-analysis. J. Psychosom. Res. 57, 35–43 (2004).
Keng, S. L., Smoski, M. J. & Robins, C. J. Effects of mindfulness on psychological health: a review of empirical studies. Clin. Psychol. Rev. 31, 1041–1056 (2011).
Teixeira, E. The effect of mindfulness meditation on painful diabetic peripheral neuropathy in adults older than 50 years. Holist. Nurs. Pract. 24, 277–283 (2010).
Zeidan, F., Grant, J. A., Brown, C. A., McHaffie, J. G. & Coghill, R. C. Mindfulness meditation-related pain relief: evidence for unique brain mechanisms in the regulation of pain. Neurosci. Lett. 520, 165–173 (2012).
Zeidan, F. et al. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J. Neurosci. 31, 5540–5548 (2011).
Davidson, R. J. & Goleman, D. J. The role of attention in meditation and hypnosis: a psychobiological perspective on transformations of consciousness. Int. J. Clin. Exp. Hypn. 25, 291–308 (1977).
Carmody, J. & Baer, R. A. Relationships between mindfulness practice and levels of mindfulness, medical and psychological symptoms and well-being in a mindfulness-based stress reduction program. J. Behav. Med. 31, 23–33 (2008).
Garland, E. L. et al. Therapeutic mechanisms of a mindfulness-based treatment for IBS: effects on visceral sensitivity, catastrophizing, and affective processing of pain sensations. J. Behav. Med. 35, 591–602 (2012).
Brown, C. A. & Jones, A. K. Meditation experience predicts less negative appraisal of≈pain: electrophysiological evidence for the involvement of anticipatory neural responses. Pain 150, 428–438 (2010).
Grant, J. A., Courtemanche, J. & Rainville, P. A non-elaborative mental stance and decoupling of executive and pain-related cortices predicts low pain sensitivity in Zen meditators. Pain 152, 150–156 (2011).
Holzel, B. K. et al. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. 191, 36–43 (2011).
Fell, J., Axmacher, N. & Haupt, S. From alpha to gamma: electrophysiological correlates of meditation-related states of consciousness. Med. Hypotheses 75, 218–224 (2010).
Ives-Deliperi, V. L., Solms, M. & Meintjes, E. M. The neural substrates of mindfulness: an fMRI investigation. Soc. Neurosci. 6, 231–242 (2011).
Craig, A. D. How do you feel—now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70 (2009).
Critchley, H. D., Wiens, S., Rotshtein, P., Ohman, A. & Dolan, R. J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 7, 189–195 (2004).
Lutz, A., McFarlin, D. R., Perlman, D. M., Salomons, T. V. & Davidson, R. J. Altered anterior insula activation during anticipation and experience of painful stimuli in expert meditators. Neuroimage 64, 538–546 (2013).
Grant, J. A., Courtemanche, J., Duerden, E. G., Duncan, G. H. & Rainville, P. Cortical thickness and pain sensitivity in zen meditators. Emotion 10, 43–53 (2010).
Rainville, P., Carrier, B., Hofbauer, R. K., Bushnell, M. C. & Duncan, G. H. Dissociation of sensory and affective dimensions of pain using hypnotic modulation. Pain 82, 159–171 (1999).
May, A. Chronic pain may change the structure ofxthe brain. Pain 137, 7–15 (2008).
Cahn, B. R. & Polich, J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol. Bull. 132, 180–211 (2006).
Aftanas, L. I. & Golocheikine, S. A. Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: high-resolution EEG investigation of meditation. Neurosci. Lett. 310, 57–60 (2001).
Barnhofer, T. et al. Effects of meditation on frontal alpha-asymmetry in previously suicidal individuals. Neuroreport 18, 709–712 (2007).
Chiesa, A. & Serretti, A. A systematic review of neurobiological and clinical features of mindfulness meditations. Psychol. Med. 40, 1239–1252 (2010).
Davidson, R. J. et al. Alterations in brain and immune function produced by mindfulness meditation. Psychosom. Med. 65, 564–570 (2003).
Davidson, R. J., Ekman, P., Saron, C. D., Senulis, J. A. & Friesen, W. V. Approach-withdrawal and cerebral asymmetry: emotional expression and brain physiology. I. J. Pers. Soc. Psychol. 58, 330–341 (1990).
Plow, E. B., Pascual-Leone, A. & Machado, A. Brain stimulation in the treatment of chronic neuropathic and non-cancerous pain. J. Pain 13, 411–424 (2012).
O'Connell, N. E., Wand, B. M., Marston, L., Spencer, S. & Desouza, L. H. Non-invasive brain stimulation techniques for chronic pain. A report of a Cochrane systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 47, 309–326 (2011).
Marlow, N. M., Bonilha, H. S. & Short, E. B. Efficacy of transcranial direct current stimulation and repetitive transcranial magnetic stimulation for treating fibromyalgia syndrome: a systematic review. Pain Pract. 13, 131–145 (2013).
Medeiros, L. F. et al. Neurobiological effects of transcranial direct current stimulation: a review. Front. Psychiatry 3, 110 (2012).
Moreno-Duarte, I. et al. Targeted therapies using electrical and magnetic neural stimulation for the treatment of chronic pain in spinal cord injury. Neuroimage 85, 1003–1013 (2013).
Leung, A. et al. rTMS for suppressing neuropathic pain: a meta-analysis. J. Pain 10, 1205–1216 (2009).
Lefaucheur, J. P. et al. Analgesic effects of repetitive transcranial magnetic stimulation of the motor cortex in neuropathic pain: influence of theta burst stimulation priming. Eur. J. Pain 16, 1403–1413 (2012).
Wrigley, P. J. et al. Longstanding neuropathic pain after spinal cord injury is refractory to transcranial direct current stimulation: a randomized controlled trial. Pain 154, 2178–2184 (2013).
Siebner, H. R. et al. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J. Neurosci. 24, 3379–3385 (2004).
Priori, A., Hallett, M. & Rothwell, J. C. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimul. 2, 241–245 (2009).
Jensen, M. P. et al. Effects of non-pharmacological pain treatments on brain states. Clin. Neurophysiol. 124, 2016–2024 (2013).
Zaghi, S., Heine, N. & Fregni, F. Brain stimulation for the treatment of pain: a review of costs, clinical effects, and mechanisms of treatment for three different central neuromodulatory approaches. J. Pain Manag. 2, 339–352 (2009).
Jasper, H. H. The ten–twenty electrode system of the International Federation. Electroencephalogr. Clin. Neurophysiol. 10, 371–375 (1958).
Sime, A. Case study of trigeminal neuralgia using neurofeedback and peripheral biofeedback. J. Neurother. 8, 59–71 (2004).
Caro, X. J. & Winter, E. F. EEG biofeedback treatment improves certain attention and somatic symptoms in fibromyalgia: a pilot study. Appl. Psychophysiol. Biofeedback 36, 193–200 (2011).
Mathew, A., Mishra, H. & Kumaraiah, V. Alpha feedback in the treatment of tension headache. J. Pers. Clin. Stud. 3, 17–22 (1987).
Cohen, M. J., McArthur, D. L. & Rickles, W. H. Comparison of four biofeedback treatments for migraine headache: physiological and headache variables. Psychosom. Med. 42, 463–480 (1980).
Andreychuk, T. & Skriver, C. Hypnosis and biofeedback in the treatment of migraine headache. Int. J. Clin. Exp. Hypn. 23, 172–183 (1975).
Kayiran, S., Dursun, E., Dursun, N., Ermutlu, N. & Karamursel, S. Neurofeedback intervention in fibromyalgia syndrome; a randomized, controlled, rater blind clinical trial. Appl. Psychophysiol. Biofeedback 35, 293–302 (2010).
Jensen, M. P. et al. Steps toward developing an EEG biofeedback treatment for chronic pain. Appl. Psychophysiol. Biofeedback 38, 101–108 (2013).
deCharms, R. C. et al. Control over brain activation and pain learned by using real-time functional MRI. Proc. Natl Acad. Sci. USA 102, 18626–18631 (2005).
Jensen, M. P., Sherline, L. H., Hakimian, S. & Fregni, F. Neuromodulatory approaches for chronic pain management: research findings and clinical implications. J. Neurother. 13, 196–213 (2009).
Bromm, B. & Lorenz, J. Neurophysiological evaluation of pain. Electroencephalogr. Clin. Neurophysiol. 107, 227–253 (1998).
Chen, A. C. Human brain measures of clinical pain: a review. I. Topographic mappings. Pain 54, 115–132 (1993).
Chen, A. C. New perspectives in EEG/MEG brain mapping and PET/fMRI neuroimaging of human pain. Int. J. Psychophysiol. 42, 147–159 (2001).
Sarnthein, J., Stern, J., Aufenberg, C., Rousson, V. & Jeanmonod, D. Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain 129, 55–64 (2006).
Llinas, R., Urbano, F. J., Leznik, E., Ramirez, R. R. & van Marle, H. J. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci. 28, 325–333 (2005).
Jensen, M. P. et al. Brain EEG activity correlates of chronic pain in persons with spinal cord injury: clinical implications. Spinal Cord 51, 55–58 (2013).
Acknowledgements
This work was supported by grants R01 HD070973 and R21 HD058049 from the NIH, awarded to M.P.J.
Author information
Authors and Affiliations
Contributions
M.P.J. and M.A.D. researched data for the article. All three authors made substantial contributions to discussions of the content, writing the article, and review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M.P.J. has published two books on the topic of hypnosis for chronic pain management (Hypnosis for Chronic Pain Management: Therapist Guide and Hypnosis for Chronic Pain Management: Workbook, both published by Oxford University Press), and receives royalties from the sales of these books. M.A.D. and J.M. declare no competing interests.
Rights and permissions
About this article
Cite this article
Jensen, M., Day, M. & Miró, J. Neuromodulatory treatments for chronic pain: efficacy and mechanisms. Nat Rev Neurol 10, 167–178 (2014). https://doi.org/10.1038/nrneurol.2014.12
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2014.12
This article is cited by
-
A novel theta-controlled vibrotactile brain–computer interface to treat chronic pain: a pilot study
Scientific Reports (2024)
-
The Effects of Mindfulness Interventions on Fibromyalgia in Adults aged 65 and Older: A Window to Effective Therapy
Journal of Clinical Psychology in Medical Settings (2023)
-
Controlled activation of cortical astrocytes modulates neuropathic pain-like behaviour
Nature Communications (2022)
-
EEG theta and beta bands as brain oscillations for different knee osteoarthritis phenotypes according to disease severity
Scientific Reports (2022)
-
Efficacy evaluation of neurofeedback applied for treatment of central neuropathic pain using machine learning
SN Applied Sciences (2021)