Abstract
Alois Alzheimer first imaged amyloid plaques in 1906 by examining dead tissue under the microscope, but their clinical significance has remained undetermined. Now, nearly a century later, investigators are beginning to image amyloid plaques in living brains using both positron emission tomography and MRI. In this article, we review the studies that report on these recent technical advances, and discuss their potential importance in clarifying the diagnostic and pathogenic relevance of amyloid plaques to Alzheimer's disease.
Key Points
-
Alzheimer's disease (AD) is emerging as one of the most common brain disorders, and considerable progress towards elucidating its molecular biology has been made in the past few years
-
Early studies indicated that insoluble species of amyloid β (Aβ) were most toxic to neurons, but recent studies in transgenic mice indicate that the soluble species of Aβ are the key neurotoxins in AD
-
Postmortem studies have provided a preliminary map of amyloid plaque localization and progression, but the ultimate goal is to image amyloid plaques in living patients
-
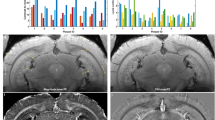
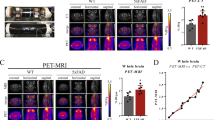
Radiolabeled ligands have been developed to enable amyloid plaques to be visualized by positron emission tomography, and this has been successfully accomplished in humans
-
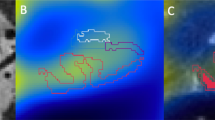
MRI is safer than positron emission tomography, and should also have better spatial resolution; to date, its potential has only been explored in transgenic mice, and numerous technical hurdles need to be overcome before it can be applied to humans
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Alzheimer A (1906) Uber einen eigenartigen schweren erkrankugundgsprozes der Hirnrinde. Neurologisches Centralblatt 23: 1129–1136
Glenner GG and Wong CW (1984) Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun 122: 1131–1135
Selkoe DJ and Podlisny MB (2002) Deciphering the genetic basis of Alzheimer's disease. Annu Rev Genomics Hum Genet 3: 67–99
Duff K (2001) Transgenic mouse models of Alzheimer's disease: phenotype and mechanisms of pathogenesis. Biochem Soc Symp 67: 195–202
Mucke L et al. (2000) High-level neuronal expression of Aβ1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci 20: 4050–4058
Pearson RC et al. (1985) Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci USA 82: 4531–4534
Price JL et al. (1991) The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer's disease. Neurobiol Aging 12: 295–312
Braak H and Braak E (1997) Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging 18: 351–357
Thal DR et al. (2002) Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 58: 1791–1800
Schonheit B et al. (2004) Spatial and temporal relationships between plaques and tangles in Alzheimer-pathology. Neurobiol Aging 25: 697–711
Shoghi-Jadid K et al. (2002) Localization of neurofibrillary tangles and β-amyloid plaques in the brains of living patients with Alzheimer disease. Am J Geriatr Psychiatry 10: 24–35
Klunk WE et al. (2004) Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol 55: 306–319
Toyama H et al. (2005) PET imaging of brain with the β-amyloid probe, [11C]6-OH-BTA-1, in a transgenic mouse model of Alzheimer's disease. Eur J Nucl Med Mol Imaging 32: 593–600
Kung MP et al. (2004) Binding of two potential imaging agents targeting amyloid plaques in postmortem brain tissues of patients with Alzheimer's disease. Brain Res 1025: 98–105
Suemoto T et al. (2004) In vivo labeling of amyloid with BF-108. Neurosci Res 48: 65–74
Verhoeff NP et al. (2004) In-vivo imaging of Alzheimer disease β-amyloid with [11C]SB-13 PET. Am J Geriatr Psychiatry 12: 584–595
Masur DM et al. (1994) Neuropsychological prediction of dementia and the absence of dementia in healthy elderly persons. Neurology 44: 1427–1432
Jacobs DM et al. (1995) Neuropsychological detection and characterization of preclinical Alzheimer's disease. Neurology 45: 957–962
Amaral DG and Witter MP (1989) The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31: 571–591
Zhao X et al. (2001) Transcriptional profiling reveals strict boundaries between hippocampal subregions. J Comp Neurol 441: 187–196
Small SA (2001) Age-related memory decline; current concepts and future directions. Arch Neurol 58: 360–364
Small SA (2003) Imaging Alzheimer's disease. Curr Neurol Neurosci Rep 3: 385–392
Arnold SE et al. (1991) The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb Cortex 1: 103–116
Giannakopoulos P et al. (2003) Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer's disease. Neurology 60: 1495–1500
Gyure KA et al. (2001) Intraneuronal Aβ-amyloid precedes development of amyloid plaques in Down syndrome. Arch Pathol Lab Med 125: 489–492
Lue LF et al. (1999) Soluble amyloid β peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol 155: 853–862
Takahashi RH et al. (2002) Intraneuronal Alzheimer Aβ42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol 161: 1869–1879
Lee SP et al. (2004) Visualization of β-amyloid plaques in a transgenic mouse model of Alzheimer's disease using MR microscopy without contrast reagents. Magn Reson Med 52: 538–544
Benveniste H et al. (1999) Detection of neuritic plaques in Alzheimer's disease by magnetic resonance microscopy. Proc Natl Acad Sci USA 96: 14079–14084
Higuchi M et al. (2005) 19F and 1H MRI detection of amyloid β plaques in vivo. Nat Neurosci 8: 527–533
Helpern JA et al. (2004) MRI assessment of neuropathology in a transgenic mouse model of Alzheimer's disease. Magn Reson Med 51: 794–798
Jack CR Jr et al. (2004) In vivo visualization of Alzheimer's amyloid plaques by magnetic resonance imaging in transgenic mice without a contrast agent. Magn Reson Med 52: 1263–1271
Vanhoutte G et al. (2005) Noninvasive in vivo MRI detection of neuritic plaques associated with iron in APP[V717I] transgenic mice, a model for Alzheimer's disease. Magn Reson Med 53: 607–613
Wadghiri YZ et al. (2003) Detection of Alzheimer's amyloid in transgenic mice using magnetic resonance microimaging. Magn Reson Med 50: 293–302
Falangola MF et al. (2005) Histological co-localization of iron in Aβ plaques of PS/APP transgenic mice. Neurochem Res 30: 201–205
Skovronsky DM et al. (2000) In vivo detection of amyloid plaques in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA 97: 7609–7614
Sossi V and Ruth TJ (2005) Micropet imaging: in vivo biochemistry in small animals. J Neural Transm 112: 319–330
Acknowledgements
This work was supported in part by NIH grants AG08702 and AG025161.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Huddleston, D., Small, S. Technology Insight: imaging amyloid plaques in the living brain with positron emission tomography and MRI. Nat Rev Neurol 1, 96–105 (2005). https://doi.org/10.1038/ncpneuro0046
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpneuro0046
This article is cited by
-
Mild Cognitive Impairment – MCI
focus neurogeriatrie (2007)