Abstract
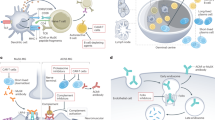
Autoimmune disorders of the neuromuscular junction remain a paradigm for our understanding of autoimmunity. Since the role of autoantibodies to acetylcholine receptors in the pathogenesis of myasthenia gravis was first recognized in the 1970s, a range of antibody-mediated disorders of the neuromuscular junction have been described, each associated with an autoantibody to a specific ligand-gated receptor, voltage-gated ion channel or related protein. In addition, antibodies to a ganglionic form of acetylcholine receptor have been detected in autoimmune forms of autonomic neuropathy. In the past few years, a role for antibodies in disorders of the CNS has begun to emerge, challenging our previous concepts regarding the blood–brain barrier and the role of the humoral immune system in CNS pathology. Although it has not yet been definitively shown that these CNS conditions are antibody-mediated, the detection of the specific antibody supports a trial of immunosuppressive therapy to which many patients appear to respond. In this article, we review the roles of antibodies to receptors and ion channels in the peripheral and central nervous systems, concentrating on the recently defined autonomic and CNS conditions and on the role of antibody measurement in diagnosis and management.
Key Points
-
The autoimmune channelopathies are neurological disorders in which the patient develops raised serum levels of antibodies to ligand-gated or voltage-gated ion channels, or related proteins
-
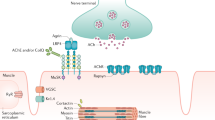
Antibodies to acetylcholine receptors are found in 80–85% of patients with generalized myasthenia gravis, and a significant proportion of the remainder have antibodies to muscle-specific kinase. Ganglionic acetylcholine-receptor antibodies are implicated in autoimmune autonomic neuropathies
-
Antibodies to other ligand-gated receptors have been detected in Rasmussen's encephalitis and neuropsychiatric systemic lupus erythematosus
-
Antibodies to voltage-gated calcium channels are associated with Lambert–Eaton myasthenic syndromes and cerebellar ataxia
-
Antibodies to voltage-gated potassium channels are found in acquired neuromyotonia and in various CNS syndromes
-
Immunomodulatory treatments are now being tested in patients with autoimmune channelopathies
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Drachman DB (1994) Myasthenia gravis. N Engl J Med 330: 1797–1810
Vincent A et al. (2001) Myasthenia gravis. Lancet 357: 2122–2128
Lindstrom JM et al. (1976) Antibody to acetylcholine receptor in myasthenia gravis: prevalence, clinical correlates, and diagnostic value. Neurology 26: 1054–1059
Toyka KV et al. (1975) Myasthenia gravis: passive transfer from man to mouse. Science 190: 397–399
Newsom-Davis J et al. (1978) Function of circulating antibody to acetylcholine receptor in myasthenia gravis: investigation by plasma exchange. Neurology 28: 266–272
Patrick J and Lindstrom J (1973) Autoimmune response to acetylcholine receptor. Science 180: 871–872
Rose NR and Bona C (1993) Defining criteria for autoimmune diseases (Witebsky's postulates revisited). Immunol Today 14: 426–430
Vincent A (2002) Unravelling the pathogenesis of myasthenia gravis. Nat Rev Immunol 2: 797–804
Riemersma S et al. (1996) Association of arthrogryposis multiplex congenita with maternal antibodies inhibiting fetal acetylcholine receptor function. J Clin Invest 98: 2358–2363
Vincent A et al. (2003) Evidence of underdiagnosis of myasthenia gravis in older people. J Neurol Neurosurg Psychiatry 74: 1105–1108
Engel AG (1984) Myasthenia gravis and myasthenic syndromes. Ann Neurol 16: 519–534
Hoch W et al. (2001) Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med 7: 365–368
Evoli A et al. (2003) Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain 126: 2304–2311
Vincent A et al. (2003) Seronegative generalised myasthenia gravis: clinical features, antibodies, and their targets. Lancet Neurol 2: 99–106
Liyanage Y et al. (2002) The agrin/muscle-specific kinase pathway: new targets for autoimmune and genetic disorders at the neuromuscular junction. Muscle Nerve 25: 4–16
Vincent A and Leite MI : Neuromuscular junction autoimmune disease—MuSK antibodies and treatments for MG. Curr Opin Neurol, in press
Vernino S et al. (2000) Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med 343: 847–855
Klein CM et al. (2003) The spectrum of autoimmune autonomic neuropathies. Ann Neurol 53: 752–758
Xu W et al. (1999) Megacystis, mydriasis, and ion channel defect in mice lacking the α3 neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci USA 96: 5746–5751
Lennon VA et al. (2003) Immunization with neuronal nicotinic acetylcholine receptor induces neurological autoimmune disease. J Clin Invest 111: 907–913
Vernino S et al. (2004) Passive transfer of autoimmune autonomic neuropathy to mice. J Neurosci 24: 7037–7042
Bien CG et al. (2005) Pathogenesis, diagnosis and treatment of Rasmussen encephalitis: a European consensus statement. Brain 128: 454–471
Freeman JM (2005) Rasmussen''s syndrome: progressive autoimmune multi-focal encephalopathy. Pediatr Neurol 32: 295–299
Rogers SW et al. (1994) Autoantibodies to glutamate receptor GluR3 in Rasmussen's encephalitis. Science 265: 648–651
McNamara JO et al. (1999) Evidence for glutamate receptor autoimmunity in the pathogenesis of Rasmussen encephalitis. Adv Neurol 79: 543–550
Wiendl H et al. (2001) GluR3 antibodies: prevalence in focal epilepsy but no specificity for Rasmussen's encephalitis. Neurology 57: 1511–1514
Mantegazza R et al. (2002) Antibodies against GluR3 peptides are not specific for Rasmussen's encephalitis but are also present in epilepsy patients with severe, early onset disease and intractable seizures. J Neuroimmunol 131: 179–185
Watson R et al. (2004) Absence of antibodies to glutamate receptor type 3 (GluR3) in Rasmussen encephalitis Neurology 63: 43–50
[No authors listed] 1999 The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 42: 599–608
DeGiorgio LA et al. (2001) A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med 7: 1189–1193
Kowal C et al. (2004) Cognition and immunity; antibody impairs memory. Immunity 21: 179–188
Husebye ES et al. (2005) Autoantibodies to a NR2A peptide of the glutamate/NMDA receptor in sera of patients with systemic lupus erythematosus. Ann Rheum Dis 64: 1210–1213
Omdal R et al. (2005) Neuropsychiatric disturbances in SLE are associated with antibodies against NMDA receptors. Eur J Neurol 12: 392–398
O'Neill JH et al. (1988) The Lambert–Eaton myasthenic syndrome. A review of 50 cases. Brain 111: 577–596
Nakao YK et al. (2002) Seronegative Lambert–Eaton myasthenic syndrome: study of 110 Japanese patients. Neurology 59: 1773–1775
Motomura M et al. (1995) An improved diagnostic assay for Lambert–Eaton myasthenic syndrome. J Neurol Neurosurg Psychiatry 58: 85–87
Lennon VA et al. (1995) Calcium-channel antibodies in the Lambert–Eaton syndrome and other paraneoplastic syndromes. N Engl J Med 332: 1467–1474
Mareska M and Gutmann L (2004) Lambert–Eaton myasthenic syndrome. Semin Neurol 24: 149–153
Lang B et al. (1983) Antibodies to motor nerve terminals: an electrophysiological study of a human myasthenic syndrome transferred to mouse. J Physiol 344: 335–345
Waterman SA et al. (1997) Effect of Lambert–Eaton myasthenic syndrome antibodies on autonomic neurons in the mouse. Ann Neurol 42: 147–156
Graus F et al. (2002) P/Q type calcium-channel antibodies in paraneoplastic cerebellar degeneration with lung cancer. Neurology 59: 764–766
Pinto A et al. (1998) Human autoantibodies specific for the α1A calcium channel subunit reduce both P-type and Q-type calcium currents in cerebellar neurons. Proc Natl Acad Sci USA 95: 8328–8333
Newsom-Davis J and Mills KR (1993) Immunological associations of acquired neuromyotonia (Isaacs' syndrome). Report of five cases and literature review. Brain 116: 453–469
Hart IK et al. (2002) Phenotypic variants of autoimmune peripheral nerve hyperexcitability. Brain 125: 1887–1895
Tomimitsu H et al. (2004) Mechanism of action of voltage-gated K+ channel antibodies in acquired neuromyotonia. Ann Neurol 56: 440–444
Shillito P et al. (1995) Acquired neuromyotonia: evidence for autoantibodies directed against K+ channels of peripheral nerves. Ann Neurol 38: 714–722
Liguori R et al. (2001) Morvan's syndrome: peripheral and central nervous system and cardiac involvement with antibodies to voltage-gated potassium channels. Brain 124: 2417–2426
Barber PA et al. (2000) Morvan's syndrome associated with voltage-gated K+ channel antibodies. Neurology 54: 771–772
Lee EK et al. (1998) Morvan's fibrillary chorea: a paraneoplastic manifestation of thymoma. J Neurol Neurosurg Psychiatry 65: 857–862
Gultekin SH et al. (2000) Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain 123: 1481–1494
Dalmau J et al. (2004) Clinical analysis of anti-Ma2-associated encephalitis. Brain 127: 1831–1844
Vincent A et al. (2004) Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain 127: 701–712
Thieben MJ et al. (2004) Potentially reversible autoimmune limbic encephalitis with neuronal potassium channel antibody. Neurology 62: 1177–1182
Pozo-Rosich P et al. (2003) Voltage-gated potassium channel antibodies in limbic encephalitis. Ann Neurol 54: 530–533
Buckley C et al. (2001) Potassium channel antibodies in two patients with reversible limbic encephalitis. Ann Neurol 50: 73–78
Schott JM et al. (2003) Amnesia, cerebral atrophy, and autoimmunity. Lancet 361: 1266
McKnight K et al.: Serum antibodies in epilepsy and seizure-associated disorders. Neurology, in press
Smart SL et al. (1998) Deletion of the K(V)1.1 potassium channel causes epilepsy in mice. Neuron 20: 809–819
Kullmann DM and Hanna MG (2002) Neurological disorders caused by inherited ion-channel mutations. Lancet Neurol 1: 157–166
Kawaguchi N et al. (2004) The Study Group for Myasthenia Gravis in Japan. Treatment and outcome of myasthenia gravis: retrospective multi-center analysis of 470 Japanese patients, 1999–2000. J Neurol Sci 224: 43–47
Keesey JC (2004) Clinical evaluation and management of myasthenia gravis. Muscle Nerve 29: 484–505
Sandroni P et al. (2005) Pyridostigmine for treatment of neurogenic orthostatic hypertension—a follow-up survey study. Clin Auton Res 15: 51–53
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Angela Vincent and the Department of Clinical Neurology receive payments, royalties and consultancy fees from RSR Ltd who market, and Athena Diagnostics who perform, some of the antibody assays mentioned in this review.
Rights and permissions
About this article
Cite this article
Buckley, C., Vincent, A. Autoimmune channelopathies. Nat Rev Neurol 1, 22–33 (2005). https://doi.org/10.1038/ncpneuro0033
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpneuro0033
This article is cited by
-
Autoimmune cardiac channelopathies: the heart of the matter
Nature Reviews Cardiology (2017)
-
Untargeted screening for novel autoantibodies with prognostic value in first-episode psychosis
Translational Psychiatry (2017)
-
Diagnostisches Management von Autoimmunenzephalitiden
Zeitschrift für Epileptologie (2015)
-
Multiple Sklerose – eine Kanalopathie?
Der Nervenarzt (2009)
-
Antibodies to AChR, MuSK and VGKC in a patient with myasthenia gravis and Morvan's syndrome
Nature Clinical Practice Neurology (2007)