Abstract
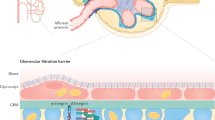
In the past decade, our understanding of the role of podocytes in the function of the glomerular filtration barrier, and of the role of podocyte injury in the pathogenesis of proteinuric kidney disease, has substantially increased. Landmark genetic studies identified mutations in genes expressed by podocytes as a cause of albuminuria and nephrotic syndrome, leading to breakthrough discoveries from many laboratories. These discoveries contributed to a dramatic change in our view of the glomerular filtration barrier of the kidney and of the role of podocyte injury in the development of albuminuria and progressive kidney disease. In the past several years, studies have demonstrated that podocyte injury is a major cause of marked albuminuria and nephrotic syndrome, and have confirmed that podocytes are important for the maintenance of an intact glomerular filtration barrier. An essential role of loss of these cells in the pathogenesis of glomerulosclerosis and progressive proteinuric kidney disease has also been identified. In this Review, we discuss the importance of podocytes for the maintenance of an intact glomerular filtration barrier and their role in albumin handling.
Key Points
-
Podocytes are highly dynamic, terminally differentiated cells that interact with the glomerular basement membrane (GBM) and communicate through signalling at the slit diaphragm
-
The glomerular filtration barrier is composed of podocytes, the GBM and endothelial cells; damage to any of these layers might result in albuminuria
-
Podocyte integrity is essential for maintenance of an intact glomerular filtration barrier and podocyte injury is a major cause of marked albuminuria
-
Several pathogenic mechanisms that are involved in podocyte injury lead to ultrastructural changes in podocytes (that is, podocyte foot process effacement) and proteinuria
-
In the past few years, exciting advances in technology have enabled visualization of the ultrastructure of living podocytes
-
These advances have paved the way for an entirely new field of research aimed at understanding the mechanisms of podocyte foot process effacement
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Haraldsson, B., Nyström, J. & Deen, W. M. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 88, 451–487 (2008).
Pavenstädt, H., Kriz, W. & Kretzler, M. Cell biology of the glomerular podocyte. Physiol. Rev. 83, 253–307 (2003).
Miner, J. H., Go, G., Cunningham, J., Patton, B. L. & Jarad, G. Transgenic isolation of skeletal muscle and kidney defects in laminin β2 mutant mice: implications for Pierson syndrome. Development 133, 967–975 (2006).
Abrahamson, D. R., Hudson, B. G., Stroganova, L., Borza, D. B. & St John, P. L. Cellular origins of type IV collagen networks in developing glomeruli. J. Am. Soc. Nephrol. 20, 1471–1479 (2009).
Eremina, V. & Quaggin, S. E. The role of VEGF-A in glomerular development and function. Curr. Opin. Nephrol. Hypertens. 13, 9–15 (2004).
Satchell, S. C., Anderson, K. L. & Mathieson, P. W. Angiopoietin 1 and vascular endothelial growth factor modulate human glomerular endothelial cell barrier properties. J. Am. Soc. Nephrol. 15, 566–574 (2004).
Satchell, S. C. et al. Human podocytes express angiopoietin 1, a potential regulator of glomerular vascular endothelial growth factor. J. Am. Soc. Nephrol. 13, 544–550 (2002).
Akilesh, S. et al. Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc. Natl Acad. Sci. USA 105, 967–972 (2008).
Farquhar, M. G., Wissig, S. L. & Palade, G. E. Glomerular permeability. I. Ferritin transfer across the normal glomerular capillary wall. J. Exp. Med. 113, 47–66 (1961).
James, J. A. & Ashworth, C. T. Some features of glomerular filtration and permeability revealed by electron microscopy after intraperitoneal injection of dextran in rats. Am. J. Pathol. 38, 515–525 (1961).
Benzing, T. Signaling at the slit diaphragm. J. Am. Soc. Nephrol. 15, 1382–1391 (2004).
Huber, T. B. & Benzing, T. The slit diaphragm: a signaling platform to regulate podocyte function. Curr. Opin. Nephrol. Hypertens. 14, 211–216 (2005).
Ly, J., Alexander, M. & Quaggin, S. E. A podocentric view of nephrology. Curr. Opin. Nephrol. Hypertens. 13, 299–305 (2004).
Mundel, P. & Shankland, S. J. Podocyte biology and response to injury. J. Am. Soc. Nephrol. 13, 3005–3015 (2002).
Kriz, W., Gretz, N., & Lemley, K. V. Progression of glomerular diseases: is the podocyte the culprit? Kidney Int. 54, 687–697 (1998).
Farquhar, M. G. & Palade, G. E. Glomerular permeability. II. Ferritin transfer across the glomerular capillary wall in nephrotic rats. J. Exp. Med. 114, 699–716 (1961).
Caulfield, J. P. & Farquhar, M. G. The permeability of glomerular capillaries of aminonuceoside nephrotic rats to graded dextrans. J. Exp. Med. 142, 61–83 (1975).
Rodewald, R. & Karnovsky, M. J. Porous substructure of the glomerular slit diaphragm in the rat and mouse. J. Cell Biol. 60, 423–433 (1974).
Ryan, G. B. & Karnovsky, M. J. An ultrastructural study of the mechanisms of proteinuria in aminonucleoside nephrosis. Kidney Int. 8, 219–232 (1975).
Tojo, A. & Endou, H. Intrarenal handling of proteins in rats using fractional micropuncture technique. Am. J. Physiol. 263, F601–F606 (1992).
Schermer, B. & Benzing, T. Lipid-protein interactions along the slit diaphragm of podocytes. J. Am. Soc. Nephrol. 20, 473–478 (2009).
Kestilä, M. et al. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol. Cell 1, 575–582 (1998).
Holzman, L. B. et al. Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int. 56, 1481–1491 (1999).
Ruotsalainen, V. et al. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc. Natl Acad. Sci. USA 96, 7962–7967 (1999).
Huber, T. B., Kottgen, M., Schilling, B., Walz, G. & Benzing, T. Interaction with podocin facilitates nephrin signaling. J. Biol. Chem. 276, 41543–41546 (2001).
Gerke, P., Huber, T. B., Sellin, L., Benzing, T. & Walz, G. Homodimerization and heterodimerization of the glomerular podocyte proteins nephrin and NEPH1. J. Am. Soc. Nephrol. 14, 918–926 (2003).
Huber, T. B. et al. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol. Cell. Biol. 23, 4917–4928 (2003).
Huber, T. B. et al. The carboxyl terminus of Neph family members binds to the PDZ domain protein zonula occludens-1. J. Biol. Chem. 278, 13417–13421 (2003).
Huber, T. B. et al. Molecular basis of the functional podocin–nephrin complex: mutations in the NPHS2 gene disrupt nephrin targeting to lipid raft microdomains. Hum. Mol. Genet. 12, 3397–3405 (2003).
Verma, R. et al. Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin. J. Biol. Chem. 278, 20716–20723 (2003).
Verma, R. et al. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin. Invest. 116, 1346–1359 (2006).
Jones, N. et al. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440, 818–823 (2006).
Kerjaschki, D. Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J. Clin. Invest. 108, 1583–1587 (2001).
Johnstone, D. B. & Holzman, L. B. Clinical impact of research on the podocyte slit diaphragm. Nat. Clin. Pract. Nephrol. 2, 271–282 (2006).
Johnson, R. I., Sedgwick, A., D' Souza-Schorey, C. & Cagan, R. L. Role for a Cindr-Arf6 axis in patterning emerging epithelia. Mol. Biol. Cell 22, 4513–4526 (2011).
Bao, S. & Cagan, R. Preferential adhesion mediated by hibris and roughest regulates morphogenesis and patterning in the Drosophila eye. Dev. Cell 8, 925–935 (2005).
Höhne, M. et al. The BAR domain protein PICK1 regulates cell recognition and morphogenesis by interacting with Neph proteins. Mol. Cell. Biol. 31, 3241–3251 (2011).
Sugie, A., Umetsu, D., Yasugi, T., Fischback, K. F. & Tabata, T. Recognition of pre- and postsynaptic neurons via nephrin/NEPH1 homologs is a basis for the formation of the Drosophila retinotopic map. Development 137, 3303–3313 (2010).
Ramos, R. G. et al. The irregular chiasm C-roughest locus of Drosophila, which affects axonal projections and programmed cell death, encodes a novel immunoglobulin-like protein. Genes Dev. 7, 2533–2547 (1993).
Strünkelnberg, M. et al. Rst and its paralogue kirre act redundantly during embryonic muscle development in Drosophila. Development 128, 4229–4239 (2001).
Weavers, H. et al. The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature 457, 322–326 (2009).
Zhuang, S. et al. Sns and Kirre, the Drosophila orthologs of Nephrin and Neph1, direct adhesion, fusion and formation of a slit diaphragm-like structure in insect nephrocytes. Development 136, 2335–2344 (2009).
Cagan, R. L. The Drosophila nephrocyte. Curr. Opin. Nephrol. Hypertens. 20, 409–415 (2011).
Fischbach, K. F. et al. The irre cell recognition module (IRM) proteins. J. Neurogenet. 23, 48–67 (2009).
Tryggvason, K. Unraveling the mechanisms of glomerular ultrafiltration: nephrin, a key component of the slit diaphragm. J. Am. Soc. Nephrol. 10, 2440–2445 (1999).
Salmon, A. H. et al. Evidence for restriction of fluid and solute movement across the glomerular capillary wall by the subpodocyte space. Am. J. Physiol. Renal. Physiol. 293: F1777–F1786 (2007).
Smithies, O. Why the kidney glomerulus does not clog: a gel permeation/diffusion hypothesis of renal function. Proc. Natl Acad. Sci. USA 100, 4108–4113 (2003).
Lazzara, M. J. & Deen, W. M. Model of albumin reabsorption in the proximal tubule. Am. J. Physiol. Renal. Physiol. 292: F430–F439 (2007).
Deen, W. M. & Lazzara, M. J. Glomerular filtration of albumin: how small is the sieving coefficient? Kidney Int. Suppl. S63–S64 (2004).
Hausmann, R. et al. Electrical forces determine glomerular permeability. J. Am. Soc. Nephrol. 21, 2053–2058 (2010).
Hildebrandt, F. Genetic kidney diseases. Lancet 375, 1287–1295 (2010).
Machuca, E., Benoit, G. & Antignac, C. Genetics of nephrotic syndrome: connecting molecular genetics to podocyte physiology. Hum. Mol. Genet. 18, R185–R194 (2009).
Moller, C. C., Pollak, M. R. & Reiser, J. The genetic basis of human glomerular disease. Adv. Chronic Kidney Dis. 13, 166–173 (2006).
Boyer, O. et al. INF2 mutations in Charcot-Marie-Tooth disease with glomerulopathy. N. Engl. J. Med. 365, 2377–2388 (2011).
Brown, E. J. et al. Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat. Genet. 42, 72–76 (2010).
Boyer, O. et al. Mutations in INF2 are a major cause of autosomal dominant focal segmental glomerulosclerosis. J. Am. Soc. Nephrol. 22, 239–245 (2011).
Barbaux, S. et al. Donor splice-site mutations in WT1 are responsible for Frasier syndrome. Nat. Genet. 17, 467–470 (1997).
Pelletier, J. et al. Germline mutations in the Wilms' tumor suppressor gene are associated with abnormal urogenital development in Denys–Drash syndrome. Cell 67, 437–447 (1991).
Denamur, E. et al. WT1 splice-site mutations are rarely associated with primary steroid-resistant focal and segmental glomerulosclerosis. Kidney Int. 57, 1868–1872 (2000).
Hinkes, B. et al. Positional cloning uncovers mutations in PLCE1 responsible for a nephrotic syndrome variant that may be reversible. Nat. Genet. 38, 1397–1405 (2006).
Gbadegesin, R. et al. Mutations in PLCE1 are a major cause of isolated diffuse mesangial sclerosis (IDMS). Nephrol. Dial. Transplant. 23, 1291–1297 (2008).
Machuca, E. et al. Genotype-phenotype correlations in non-Finnish congenital nephrotic syndrome. J. Am. Soc. Nephrol. 21, 1209–1217 (2010).
Boute, N. et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat. Genet. 24, 349–354 (2000).
Jungraithmayr, T. C. et al. Screening for NPHS2 mutations may help predict FSGS recurrence after transplantation. J. Am. Soc. Nephrol. 22, 579–585 (2011).
Weber, S. et al. NPHS2 mutation analysis shows genetic heterogeneity of steroid-resistant nephrotic syndrome and low post-transplant recurrence. Kidney Int. 66, 571–579 (2004).
Reiser, J. et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat. Genet. 37, 739–744 (2005).
Winn, M. P. et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308, 1801–1804 (2005).
Kaplan, J. M. et al. Mutations in ACTN4, encoding α-actinin-4, cause familial focal segmental glomerulosclerosis. Nat. Genet. 24, 251–256 (2000).
Roselli, S. et al. Podocin localizes in the kidney to the slit diaphragm area. Am. J. Pathol. 160, 131–139 (2002).
Huber, T. B., Schermer, B. & Benzing, T. Podocin organizes ion channel-lipid supercomplexes: implications for mechanosensation at the slit diaphragm. Nephron Exp. Nephrol. 106, e27–e31 (2007).
Huber, T. B. et al. Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc. Natl Acad. Sci. USA 103, 17079–17086 (2006).
Goodman, M. B. et al. MEC-2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature 415, 1039–1042 (2002).
Huang, M., Gu, G., Ferguson, E. L. & Chalfie, M. A stomatin-like protein necessary for mechanosensation in C. elegans. Nature 378, 292–295 (1995).
Huber, T. B. et al. Bigenic mouse models of focal segmental glomerulosclerosis involving pairwise interaction of CD2AP, Fyn, and synaptopodin. J. Clin. Invest. 116, 1337–1345 (2006).
Dryer, S. E. & Reiser, J. TRPC6 channels and their binding partners in podocytes: role in glomerular filtration and pathophysiology. Am. J. Physiol. Renal Physiol. 299, F689–F701 (2010).
Shankland, S. J. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 69, 2131–2147 (2006).
Kriz, W., Shirato, I., Nagata, M., Lehir, M. & Lemley, K. V. The podocyte's response to stress: the enigma of foot process effacement. Am. J. Physiol. Renal Physiol. 304, F333–F347 (2012).
Clement, L. C. et al. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat. Med. 17, 117–122 (2011).
Jin, J. et al. Soluble FLT1 binds lipid microdomains in podocytes to control cell morphology and glomerular barrier function. Cell 151, 384–399 (2012).
Wei, C. et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat. Med. 17, 952–960 (2011).
Eremina, V. et al. Vascular endothelial growth factor a signaling in the podocyte–endothelial compartment is required for mesangial cell migration and survival. J. Am. Soc. Nephrol. 17, 724–735 (2006).
Eremina, V. et al. VEGF inhibition and renal thrombotic microangiopathy. N. Engl. J. Med. 358, 1129–1136 (2008).
Levine, R. J. et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 350, 672–683 (2004).
Thadhani, R. et al. Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia. Circulation 124, 940–950 (2011).
Faul, C. et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat. Med. 14, 931–938 (2008).
Jiang, L. et al. Over-expressing transient receptor potential cation channel 6 in podocytes induces cytoskeleton rearrangement through increases of intracellular Ca2+ and RhoA activation. Exp. Biol. Med. (Maywood) 236, 184–193 (2011).
Tian, D. et al. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci. Signal. 3, ra77 (2010).
Faul, C., Asanuma, K., Yanagida-Asanuma, E., Kim, K. & Mundel, P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 17, 428–437 (2007).
Shibata, S. et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat. Med. 14, 1370–1376 (2008).
Scott, R. P. et al. Podocyte-specific loss of Cdc42 leads to congenital nephropathy. J. Am. Soc. Nephrol. 23, 1149–1154 (2012).
Wang, L. et al. Mechanisms of the proteinuria induced by Rho GTPases. Kidney Int. 81, 1075–1085 (2012).
Zhu, L., Jiang, R., Aoudjit, L., Jones, N. & Takano, T. Activation of RhoA in podocytes induces focal segmental glomerulosclerosis. J. Am. Soc. Nephrol. 22, 1621–1630 (2011).
Laplante, M. & Sabatini, D. M. mTOR signaling in growth control and disease. Cell 149, 274–293 (2012).
Ciná, D. P. et al. Inhibition of MTOR disrupts autophagic flux in podocytes. J. Am. Soc. Nephrol. 23, 412–420 (2012).
Letavernier, E. & Legendre, C. mToR inhibitors-induced proteinuria: mechanisms, significance, and management. Transplant. Rev. (Orlando) 22, 125–130 (2008).
Inoki, K. et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J. Clin. Invest. 121, 2181–2196 (2011).
Gödel, M. et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J. Clin. Invest. 121, 2197–2209 (2011).
Brahler, S. et al. Intrinsic proinflammatory signaling in podocytes contributes to podocyte damage and prolonged proteinuria. Am. J. Physiol. Renal. Physiol. 303, F1473–F1485 (2012).
Mathieson, P. W. Proteinuria and immunity—an overstated relationship? N. Engl. J. Med. 359, 2492–2494 (2008).
Hussain, S. et al. Nephrin deficiency activates NF-κB and promotes glomerular injury. J. Am. Soc. Nephrol. 20, 1733–1743 (2009).
Garg, P. & Holzman, L. B. Podocytes: gaining a foothold. Exp. Cell Res. 318, 955–963 (2012).
Hirose, T. et al. An essential role of the universal polarity protein, aPKCλ, on the maintenance of podocyte slit diaphragms. PLoS ONE 4, e4194 (2009).
Huber, T. B. et al. Loss of podocyte aPKCλ/ι causes polarity defects and nephrotic syndrome. J. Am. Soc. Nephrol. 20, 798–806 (2009).
Hartleben, B. et al. Role of the polarity protein Scribble for podocyte differentiation and maintenance. PLoS ONE 7, e36705 (2012).
Hartleben, B. et al. Neph-Nephrin proteins bind the Par3–Par6–atypical protein kinase C (aPKC) complex to regulate podocyte cell polarity. J. Biol. Chem. 283, 23033–23038 (2008).
Brinkkoetter, P. T. et al. Cyclin I activates Cdk5 and regulates expression of Bcl-2 and Bcl-XL in postmitotic mouse cells. J. Clin. Invest. 119, 3089–3101 (2009).
Brinkkoetter, P. T. et al. p35, the non-cyclin activator of Cdk5, protects podocytes against apoptosis in vitro and in vivo. Kidney Int. 77, 690–699 (2010).
Susztak, K., Raff, A. C., Schiffer, M. & Böttinger, E. P. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55, 225–233 (2006).
Schiffer, M. et al. Apoptosis in podocytes induced by TGF-β and Smad7. J. Clin. Invest. 108, 807–816 (2001).
Wharram, B. L. et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J. Am. Soc. Nephrol. 16, 2941–2952 (2005).
Cheung, Z. H., Gong, K. & Ip, N. Y. Cyclin-dependent kinase 5 supports neuronal survival through phosphorylation of Bcl-2. J. Neurosci. 28, 4872–4877 (2008).
Najafian, B., Alpers, C. E. & Fogo, A. B. Pathology of human diabetic nephropathy. Contrib. Nephrol. 170, 36–47 (2011).
Hara, M., Yanagihara, T. & Kihara, I. Cumulative excretion of urinary podocytes reflects disease progression in IgA nephropathy and Schönlein–Henoch purpura nephritis. Clin. J. Am. Soc. Nephrol. 2, 231–238 (2007).
Höhne, M. et al. Light microscopic visualization of podocyte ultrastructure demonstrates oscillating glomerular contractions. Am. J. Pathol. 182, 332–338 (2012).
Grgic, I. et al. Imaging of podocyte foot processes by fluorescence microscopy. J. Am. Soc. Nephrol. 23, 785–791 (2012).
Peti-Peterdi, J. & Sipos, A. A high-powered view of the filtration barrier. J. Am. Soc. Nephrol. 21, 1835–1841 (2010).
Peti-Peterdi, J., Burford, J. L. & Hackl, M. J. The first decade of using multiphoton microscopy for high-power kidney imaging. Am. J. Physiol. Renal Physiol. 302, F227–F233 (2012).
Nakano, D. et al. Multiphoton imaging of the glomerular permeability of angiotensinogen. J. Am. Soc. Nephrol. 23, 1847–1856 (2012).
Mangos, S. & Reiser, J. Fishing for new glomerular disease-related genes. J. Am. Soc. Nephrol. 22, 1960–1962 (2011).
Mathieson, P. W. The podocyte as a target for therapies—new and old. Nat. Rev. Nephrol. 8, 52–56 (2012).
Muller, R. U. & Benzing, T. A photo shoot of proteinuria: zebrafish models of inducible podocyte damage. J. Am. Soc. Nephrol. 23, 969–971 (2012).
Kramer-Zucker, A. G., Wiessner, S., Jensen, A. M. & Drummond, I. A. Organization of the pronephric filtration apparatus in zebrafish requires Nephrin, Podocin and the FERM domain protein Mosaic eyes. Dev. Biol. 285, 316–329 (2005).
Hentschel, D. M. et al. Rapid screening of glomerular slit diaphragm integrity in larval zebrafish. Am. J. Physiol. Renal Physiol. 293, F1746–F1750 (2007).
Zhou, W. & Hildebrandt, F. Inducible podocyte injury and proteinuria in transgenic zebrafish. J. Am. Soc. Nephrol. 23, 1039–1047 (2012).
Kim, J. M. et al. CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science 23, 1298–1300 (2003).
Zenker, M. et al. Human laminin β2 deficiency causes congenital nephrosis with mesangial sclerosis and disinct eye abnormalities. Hum. Mol. Genet. 13, 2625–2632 (2004).
Mele, C. et al. MYO1E mutations and childhood familial focal segmenal glomerulosclerosis. N. Engl. J. Med. 365, 295–306 (2011).
Heeringa, S. F. et al. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J. Clin. Invest. 121, 2013–2024 (2011).
Ozaltin, F. et al. Disruption of PTPRO causes childhood-onset nephrotic syndrome. Am. J. Hum. Genet. 89, 139–147 (2011).
Habib, R. et al. The nephropathy associated with male pseudohermaphroditism and Wilms' tumor (Drash syndrome): a distinctive glomerular lesion—report of 10 cases. Clin. Nephrol. 24, 269–278 (1985).
Seri, M. et al. Mutations in MYH9 result in the May-Hegglin anomaly, and Fectner and Sebastian Syndromes. Nat. Genet. 26, 103–105 (2000).
Dreyer, S. D. et al. Mutations in LMX1B cause abnormal skeletal patterning and renal dysplasia in nail patella syndrome. Nat. Genet. 19, 47–50 (1998).
McIntosh, I. et al. Mutation analysis of LMX1B gene in nail patella syndrome patients. Am. J. Hum. Genet. 63, 1651–1658 (1998).
Vollrath, D. et al. Loss-of-function mutations in the LIM-homeodomain gene, LMX1B, in nail-patella syndrome. Hum. Mol. Genet. 7, 1091–1098 (1998).
Boerkoel, C. F. et al. Mutant chromatin remodelling protein SMARCAL1 causes Schimke immuno-osseous dysplasia. Nat. Genet. 30, 215–220 (2002).
Quinzii, C. et al. A mutation in para-hydroxybenzoate-polyprenyl transferase (COQ2) causes primary coenzyme Q10 deficiency. Am. J. Hum. Genet. 78, 345–349 (2006).
Salviati, L. et al. Infantile encephalomyopathy and nephropathy with CoQ10 deficiency: a CoQ10-responsive condition. Neurology 65, 606–608 (2005).
López, L. C. et al. Leigh syndrome with nephropathy and CoQ10 deficiency due to decaprenyl disphosphate synthase subunit 2 (PDSS2) mutations. Am. J. Hum. Genet. 79, 1125–1129 (2006).
Kurogouchi, F. et al. A case of mitochondrial cytopathy with a typical point mutation for MELAS, presenting with severe focal-segmental glomerulosclerosis as main clinical manifestation. Am. J. Nephrol. 18, 551–556 (1998).
Goto, Y., Nonaka, I. & Horai, S. A mutation in the tRNALeu(URR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature, 348, 651–653 (1990).
Balreira, A. et al. A nonsense mutation in the LIMP-2 gene associated with progressive myoclonic epilepsy and nephrotic syndrome. Hum. Mol. Genet. 17, 2238–2243 (2008).
Berkovic, S. F. et al. Array-based gene discovery with three unrelated subjects shows SCARB2/LIMP-2 deficiency causes myoclonus epilepsy and glomerulosclerosis. Am. J. Hum. Genet. 82, 673–684 (2008).
Castelletti, F. et al. Mutations in FN1 cause glomerulopathy with fibronectin deposits. Proc.Natl. Acad. Sci. USA 105, 2538–2543 (2008).
Acknowledgements
The authors' work is supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 635 [T. Benzing] and BR2955 [P. T. Brinkkoetter]). Podocyte research has benefited from the contribution of many groups all over the world and we apologize to those colleagues whose work could not be cited due to space limitations.
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article. T. Benzing and P. T. Brinkkoetter wrote the article and C. Ising reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
T. Benzing has received speaker's honoraria from Amgen, Hexal, Novartis and Otsuka. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Brinkkoetter, P., Ising, C. & Benzing, T. The role of the podocyte in albumin filtration. Nat Rev Nephrol 9, 328–336 (2013). https://doi.org/10.1038/nrneph.2013.78
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2013.78
This article is cited by
-
Downregulating lncRNA MIAT attenuates apoptosis of podocytes exposed to high glucose
Acta Diabetologica (2023)
-
The E3 ubiquitin ligase TRIM31 plays a critical role in hypertensive nephropathy by promoting proteasomal degradation of MAP3K7 in the TGF-β1 signaling pathway
Cell Death & Differentiation (2022)
-
Sestrin2 remedies podocyte injury via orchestrating TSP-1/TGF-β1/Smad3 axis in diabetic kidney disease
Cell Death & Disease (2022)
-
Right Heart Function in Cardiorenal Syndrome
Current Heart Failure Reports (2022)
-
The role of the immune system in idiopathic nephrotic syndrome
Molecular and Cellular Pediatrics (2021)