Abstract
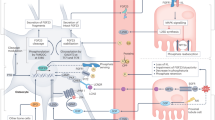
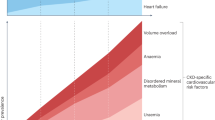
The metabolic changes that occur in patients with chronic kidney disease (CKD) have a profound influence on mineral and bone metabolism. CKD results in altered levels of serum phosphate, vitamin D, calcium, parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF-23); the increased levels of serum phosphate, PTH and FGF-23 contribute to the increased cardiovascular mortality in affected patients. FGF-23 is produced by osteocytes and osteoblasts and acts physiologically in the kidney to induce phosphaturia and inhibit the synthesis of 1,25-dihydroxyvitamin D3. PTH acts directly on osteocytes to increase FGF-23 expression. In addition, the high levels of PTH associated with CKD contribute to changes in bone remodelling that result in decreased levels of dentin matrix protein 1 and the release of low-molecular-weight fibroblast growth factors from the bone matrix, which stimulate FGF-23 transcription. A prolonged oral phosphorus load increases FGF-23 expression by a mechanism that includes local changes in the ratio of inorganic phosphate to pyrophosphate in bone. Other factors such as dietary vitamin D compounds, calcium, and metabolic acidosis all increase FGF-23 levels. This Review discusses the mechanisms by which secondary hyperparathyroidism associated with CKD stimulates bone cells to overexpress FGF-23 levels.
Key Points
-
Secondary hyperparathyroidism and increased serum fibroblast growth factor 23 (FGF-23) levels in chronic kidney disease (CKD) are intimately related; secondary hyperparathyroidism has a crucial role in increasing the expression of FGF-23
-
Parathyroid hormone (PTH) increases FGF-23 expression in osteoblast-like cells in vitro by activating protein kinase A and Wnt signalling pathways
-
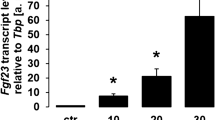
The ratio of inorganic phosphate to pyrophosphate in the bone matrix is increased in CKD, which increases FGF-23 expression
-
The loss of FGF-23 transcriptional inhibition by dentin matrix protein 1 and the release of low-molecular-weight fibroblast growth factors increase FGF-23 expression in CKD
-
FGF-23 acts on the kidney to decrease 1,25-dihydroxyvitamin D3 (1,25[OH]2D3) synthesis, which contributes to the secondary hyperparathyroidism of CKD, whereas 1,25(OH)2D3 itself increases FGF-23 transcription
-
The FGF-23-receptor complex, Klotho–fibroblast growth factor receptor 1, is downregulated in the parathyroid gland of patients with CKD, resulting in loss of FGF-23's ability to decrease PTH expression
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Meyer, R. A., Meyer, M. H. & Gray, R. W. Parabiosis suggests a humoral factor is involved in X-linked hypophosphatemia in mice. J. Bone Miner. Res. 4, 493–500 (1989).
Nesbitt, T., Coffman, T. M., Griffiths, R. & Drezner, M. K. Crosstransplantation of kidneys in normal and Hyp mice. Evidence that the Hyp mouse phenotype is unrelated to an intrinsic defect. J. Clin. Invest. 89, 1453–1459 (1992).
ADHR consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 26, 345–348 (2000).
Shimada, T. et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc. Natl Acad. Sci. USA 98, 6500–6505 (2001).
Kuro-o, M. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51 (1997).
Goetz, R. et al. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc. Natl Acad. Sci. USA 107, 407–412 (2010).
Gutierrez, O. M. et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N. Engl. J. Med. 359, 584–592 (2008).
Isakova, T. et al. Phosphorus binders and survival on hemodialysis. J. Am. Soc. Nephrol. 20, 388–396 (2009).
Ix, J. H., Shlipak, M. G., Wassel, C. L. & Whooley, M. A. Fibroblast growth factor-23 and early decrements in kidney function: the Heart and Soul Study. Nephrol. Dial. Transplant. 25, 993–997 (2009).
Silver, J., Kilav, R. & Naveh-Many, T. Mechanisms of secondary hyperparathyroidism. Am. J. Physiol. Renal Physiol. 283, F367–F376 (2002).
Komaba, H. & Fukagawa, M. The role of FGF23 in CKD—with or without Klotho. Nat. Reviews Nephrol. 8, 484–490 (2012).
Wolf, M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 82, 737–747 (2012).
Quarles, L. D. Role of FGF23 in vitamin D and phosphate metabolism: implications in chronic kidney disease. Exp. Cell Res. 318, 1040–1048 (2012).
Silver, J., Rodriguez, M. & Slatopolsky, E. FGF23 and PTH: double agents at the heart of CKD. Nephrol. Dial. Transplant. 27, 1715–1720 (2012).
Bergwitz, C. & Juppner, H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu. Rev. Med. 61, 91–104 (2010).
Slatopolsky, E. et al. On the pathogenesis of hyperparathyroidism in chronic experimental renal insufficiency in the dog. J. Clin. Invest. 50, 492–499 (1971).
Slatopolsky, E. & Bricker, N. S. The role of phosphorus restriction in the prevention of secondary hyperparathyroidism in chronic renal disease. Kidney Int. 4, 141–145 (1973).
Kilav, R., Silver, J. & Naveh-Many, T. Parathyroid hormone gene expression in hypophosphatemic rats. J. Clin. Invest. 96, 327–333 (1995).
Almaden, Y. et al. Direct effect of phosphorus on parathyroid hormone secretion from whole rat parathyroid glands in vitro. J. Bone Miner. Res. 11, 970–976 (1996).
Slatopolsky, E. et al. Phosphate restriction prevents parathyroid cell growth in uremic rats. High phosphate directly stimulates PTH secretion in vitro. J. Clin. Invest. 97, 2534–2540 (1996).
Nielsen, P. K., Feldt-Rasmusen, U. & Olgaard, K. A direct effect of phosphate on PTH release from bovine parathyroid tissue slices but not from dispersed parathyroid cells. Nephrol. Dial. Transplant. 11, 1762–1768 (1996).
Sapir-Koren, R. & Livshits, G. Bone mineralization and regulation of phosphate homeostasis. IBMS BoneKEy 8, 286–300 (2011).
Sage, A. P., Lu, J. X., Tintut, Y. & Demer, L. L. Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney Int. 79, 414–422 (2011).
Perwad, F. et al. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology 146, 5358–5364 (2005).
Hasegawa, H. et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 78, 975–980 (2010).
Shalhoub, V. et al. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J. Clin. Invest. 122, 2543–2553 (2012).
Meir, T. et al. Deletion of the vitamin D receptor specifically in the parathyroid demonstrates a limited role for the VDR in parathyroid physiology. Am. J. Physiol. Renal Physiol. 297, F1192–F1198 (2009).
Li, Y. C. et al. Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology 139, 4391–4396 (1998).
Nishida, Y. et al. Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int. 70, 2141–2147 (2006).
Ferrari, S. L., Bonjour, J. P. & Rizzoli, R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J. Clin. Endocrinol. Metab. 90, 1519–1524 (2005).
Gutierrez, O. M., Wolf, M. & Taylor, E. N. Fibroblast growth factor 23, cardiovascular disease risk factors, and phosphorus intake in the Health Professionals Follow-up Study. Clin. J. Am. Soc. Nephrol. 6, 2871–2878 (2011).
Westerberg, P. A. et al. Regulation of fibroblast growth factor-23 in chronic kidney disease. Nephrol. Dial. Transplant. 22, 3202–3207 (2007).
Isakova, T. et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 79, 1370–1378 (2011).
Ito, N. et al. Effect of acute changes of serum phosphate on fibroblast growth factor (FGF)23 levels in humans. J. Bone Miner. Metab. 25, 419–422 (2007).
Isakova, T. et al. Postprandial mineral metabolism and secondary hyperparathyroidism in early CKD. J. Am. Soc. Nephrol. 19, 615–623 (2008).
Pavik, I. et al. Secreted Klotho and FGF23 in chronic kidney disease Stage 1 to 5: a sequence suggested from a cross-sectional study. Nephrol. Dial. Transplant. 28, 352–359 (2013).
Levin, A. et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 71, 31–38 (2007).
Oliveira, R. B. et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin. J. Am. Soc. Nephrol. 5, 286–291 (2010).
Isakova, T. et al. Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol. Dial. Transplant. 26, 584–591 (2011).
Gonzalez-Parra, E. et al. Lanthanum carbonate reduces FGF23 in chronic kidney disease stage 3 patients. Nephrol. Dial. Transplant. 26, 2567–2571 (2011).
Gupta, A., Winer, K., Econs, M. J., Marx, S. J. & Collins, M. T. FGF-23 is elevated by chronic hyperphosphatemia. J. Clin. Endocrinol. Metab. 89, 4489–4492 (2004).
Yamashita, H. et al. Fibroblast growth factor-23 (FGF23) in patients with transient hypoparathyroidism: its important role in serum phosphate regulation. Endocr. J. 54, 465–470 (2007).
Araya, K. et al. A novel mutation in fibroblast growth factor 23 gene as a cause of tumoral calcinosis. J. Clin. Endocrinol. Metab. 90, 5523–5527 (2005).
Yamashita, H. et al. Fibroblast growth factor (FGF)-23 in patients with primary hyperparathyroidism. Eur. J. Endocrinol. 151, 55–60 (2004).
Tebben, P. J., Singh, R. J., Clarke, B. L. & Kumar, R. Fibroblast growth factor 23, parathyroid hormone, and 1α,25-dihydroxyvitamin D in surgically treated primary hyperparathyroidism. Mayo Clin. Proc. 79, 1508–1513 (2004).
Yuan, Q. et al. FGF-23/Klotho signaling is not essential for the phosphaturic and anabolic functions of PTH. J. Bone Miner. Res. 26, 2026–2035 (2011).
Lomashvili, K. A., Khawandi, W. & O'Neill, W. C. Reduced plasma pyrophosphate levels in hemodialysis patients. J. Am. Soc. Nephrol. 16, 2495–2500 (2005).
Alfrey, A. C. & Solomons, C. C. Bone pyrophosphate in uremia and its association with extraosseous calcification. J. Clin. Invest. 57, 700–705 (1976).
Huitema, L. F. A. et al. Entpd5 is essential for skeletal mineralization and regulates phosphate homeostasis in zebrafish. Proc. Natl Acad. Sci. USA 109, 21372–21377 (2012).
Mackenzie, N. C. W. et al. Altered bone development and an increase in FGF-23 expression in Enpp1−/− mice. PLoS ONE 7, (2012).
Lorenz-Depiereux, B., Schnabel, D., Tiosano, D., Häusler, G. & Strom, T. M. Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am. J. Hum. Genet. 86, 267–272 (2010).
Murshed, M., Harmey, D., Millan, J. L., McKee, M. D. & Karsenty, G. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev. 19, 1093–1104 (2005).
Quarles, L. D. Endocrine functions of bone in mineral metabolism regulation. J. Clin. Invest. 118, 3820–3828 (2008).
Rowe, P. S. N. Regulation of bone-renal mineral and energy metabolism: the PHEX, FGF23, DMP1, MEPE ASARM pathway. Crit. Rev. Eukaryot. Gene Expr. 22, 61–86 (2012).
Martin, A., David, V. & Quarles, L. D. Regulation and function of the Fgf23/Klotho endocrine pathways. Physiol. Rev. 92, 131–155 (2012).
Martin, A. et al. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. 25, 2551–2562 (2011).
Wohrle, S. et al. FGF receptors control vitamin D and phosphate homeostasis by mediating renal FGF-23 signaling and regulating FGF-23 expression in bone. J. Bone Miner. Metab. 26, 2486–2497 (2011).
Bonewald, L. F. Osteocyte messages from a bony tomb. Cell Metab. 5, 410–411 (2007).
The Hyp consortium. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat. Genet. 11, 130–136 (1995).
Lorenz-Depiereux, B. et al. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat. Genet. 38, 1248–1250 (2006).
Yuan, B. et al. Aberrant Phex function in osteoblasts and osteocytes alone underlies murine X-linked hypophosphatemia. J. Clin. Invest. 118, 722–734 (2008).
White, K. E. et al. Mutations that cause osteoglophonic dysplasia define novel roles for FGFR1 in bone elongation. Am. J. Hum. Genet. 76, 361–367 (2005).
Goebel, S. et al. FGF23 is a putative marker for bone healing and regeneration. J. Orthop. Res. 27, 1141–1146 (2009).
Shimada, T. et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 19, 429–435 (2004).
Krajisnik, T. et al. Fibroblast growth factor-23 regulates parathyroid hormone and 1α-hydroxylase expression in cultured bovine parathyroid cells. J. Endocrinol. 195, 125–131 (2007).
Ben Dov, I. Z. et al. The parathyroid is a target organ for FGF23 in rats. J. Clin. Invest. 117, 4003–4008 (2007).
Lavi-Moshayoff, V., Silver, J. & Naveh-Many, T. Human PTH gene regulation in vivo using transgenic mice. Am. J. Physiol. Renal Physiol. 297, F713–F719 (2009).
Galitzer, H., Ben Dov, I. Z., Silver, J. & Naveh-Many, T. Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int. 77, 211–218 (2010).
Komaba, H. et al. Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int. 77, 232–238 (2010).
Canalejo, R. et al. FGF23 fails to inhibit uremic parathyroid glands. J. Am. Soc. Nephrol. 21, 1125–1135 (2010).
Komaba, H. & Fukagawa, M. FGF23-parathyroid interaction: implications in chronic kidney disease. Kidney Int. 77, 292–298 (2010).
Hofman-Bang, J., Martuseviciene, G., Santini, M. A., Olgaard, K. & Lewin, E. Increased parathyroid expression of klotho in uremic rats. Kidney Int. 78, 1119–1127 (2010).
Kobayashi, K. et al. Regulation of plasma fibroblast growth factor 23 by calcium in primary hyperparathyroidism. Eur. J. Endocrinol. 154, 93–99 (2006).
Brown, W. W. et al. Hypophosphatemia with elevations in serum FGF23 in a child with Jansen's metaphyseal chondrodysplasia in Jansen's syndrome. J. Clin. Endocrinol. Metab. 94, 17–20 (2008).
Rhee, Y. et al. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone 49, 636–643 (2011).
Lavi-Moshayoff, V., Wasserman, G., Meir, T., Silver, J. & Naveh-Many, T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am. J. Physiol. Renal Physiol. 299, F882–F889 (2010).
Koizumi, M., Komaba, H., Nakanishi, S., Fujimori, A. & Fukagawa, M. Cinacalcet treatment and serum FGF23 levels in haemodialysis patients with secondary hyperparathyroidism. Nephrol. Dial. Transplant. 27, 784–790 (2011).
Wetmore, J. B., Liu, S., Krebill, R., Menard, R. & Quarles, L. D. Effects of cinacalcet and concurrent low-dose vitamin D on FGF23 levels in ESRD. Clin. J. Am. Soc. Nephrol. 5, 110–116 (2010).
Liu, S. et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J. Am. Soc. Nephrol. 17, 1305–1315 (2006).
Saito, H. et al. Circulating FGF-23 is regulated by 1α,25-dihydroxyvitamin D3 and phosphorus in vivo. J. Biol. Chem. 280, 2543–2549 (2005).
Kolek, O. I. et al. 1α,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G1036–G1042 (2005).
Shimada, T. et al. FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem. Biophys. Res. Commun. 314, 409–414 (2004).
Hansen, D., Rasmussen, K., Pedersen, S. M., Rasmussen, L. M. & Brandi, L. Changes in fibroblast growth factor 23 during treatment of secondary hyperparathyroidism with alfacalcidol or paricalcitol. Nephrol. Dial. Transplant. 27, 2263–2269 (2012).
Grethen, E. et al. Serum leptin, parathyroid hormone, 1,25-dihydroxyvitamin D, fibroblast growth factor 23, bone alkaline phosphatase, and sclerostin relationships in obesity. J. Clin. Endocrinol. Metab. 97, 1655–1662 (2012).
Tsuji, K., Maeda, T., Kawane, T., Matsunuma, A. & Horiuchi, N. Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1α,25-dihydroxyvitamin D3 synthesis in leptin-deficient mice. J. Bone Miner. Res. 25, 1711–1723 (2010).
Saini, R. K. et al. 1,25-Dihydroxyvitamin D3 regulation of fibroblast growth factor-23 expression in bone cells: evidence for primary and secondary mechanisms modulated by leptin and interleukin-6. Calcif. Tissue Int. 92, 339–353 (2013).
Masuyama, R. et al. Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J. Clin. Invest. 116, 3150–3159 (2006).
Shimada, T. et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am. J. Physiol. Renal Physiol. 289, F1088–F1095 (2005).
Quinn, S. J. et al. CaSR-mediated interactions between calcium and magnesium homeostasis in mice. Am. J. Physiol. Endocrinol. Metab. 304, E724–E733 (2013).
Rodriguez-Ortiz, M. E. et al. Calcium deficiency reduces circulating levels of FGF23. J. Am. Soc. Nephrol. 23, 1190–1197 (2012).
Chen, C. D., Podvin, S., Gillespie, E., Leeman, S. E. & Abraham, C. R. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM 17. Proc. Natl Acad. Sci. USA 104, 19796–19801 (2007).
Matsumura, Y. et al. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem. Biophys. Res. Commun. 242, 626–630 (1998).
Imura, A. et al. A-Klotho as a regulator of calcium homeostasis. Science 316, 1615–1618 (2007).
Ichikawa, S. et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J. Clin. Invest. 117, 2684–2691 (2007).
Brownstein, C. A. et al. A translocation causing increased α-Klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc. Natl Acad. Sci. USA 105, 3455–3460 (2008).
Smith, R. C. et al. Circulating αKlotho influences phosphate handling by controlling FGF23 production. J. Clin. Invest. 122, 4710–4715 (2012).
Juppner, H. & Wolf, M. αKlotho: FGF23 coreceptor and FGF23-regulating hormone. J. Clin. Invest. 122, 4336–4339 (2012).
Krieger, N. S., Culbertson, C. D., Kyker-Snowman, K. & Bushinsky, D. A. Metabolic acidosis increases fibroblast growth factor 23 in neonatal mouse bone. Am. J. Physiol. Renal Physiol. 303, F431–F436 (2012).
Farrow, E. G. et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc. Natl Acad. Sci. USA 108, E1146–E1155 (2011).
Takeda, Y. et al. Effect of intravenous saccharated ferric oxide on serum FGF23 and mineral metabolism in hemodialysis patients. Am. J. Nephrol. 33, 421–426 (2011).
Carrillo-Lopez, N. et al. Indirect regulation of PTH by estrogens may require FGF23. J. Am. Soc. Nephrol. 20, 2009–2017 (2009).
Brandenburg, V. M. et al. Serological cardiovascular and mortality risk predictors in dialysis patients receiving sevelamer: a prospective study. Nephrol. Dial. Transplant. 25, 2672–2679 (2010).
Shimada, T. et al. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology 143, 3179–3182 (2002).
Yamazaki, Y. et al. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J. Clin. Endocrinol. Metab. 87, 4957–4960 (2002).
Collins, M. T. et al. Renal phosphate wasting in fibrous dysplasia of bone is part of a generalized renal tubular dysfunction similar to that seen in tumor-induced osteomalacia. J. Bone Miner. Res. 16, 806–813 (2001).
Yu, X., Sabbagh, Y., Davis, S. I., Demay, M. B. & White, K. E. Genetic dissection of phosphate- and vitamin D-mediated regulation of circulating Fgf23 concentrations. Bone 36, 971–977 (2005).
Kato, K. et al. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J. Biol. Chem. 281, 18370–18377 (2006).
Yu, X. & White, K. E. FGF23 and disorders of phosphate homeostasis. Cytokine Growth Factor Rev. 16, 221–232 (2005).
Bhattacharyya, N. et al. Mechanism of FGF23 processing in fibrous dysplasia. J. Bone Miner. Res. 27, 1132–1141 (2012).
Faul, C. et al. FGF23 induces left ventricular hypertrophy. J. Clin. Invest. 121, 4393–4408 (2011).
Acknowledgements
The authors' work is supported the Harold and Ethel Pupkewitz Fund for Renal Research and Amgen. T. Naveh-Many's work is also supported by the Israel Science Foundation.
Author information
Authors and Affiliations
Contributions
J. Silver wrote the article. J. Silver and T. Naveh-Many contributed equally to researching data for the article, discussions of the content, and review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J. Silver and T. Naveh-Many have received a research grant from Amgen. J. Silver has received honoraria and lecture fees from Amgen, Fresenius, KAI Pharmaceuticals, Shire, and Teva Pharmaceutical Industries.
Rights and permissions
About this article
Cite this article
Silver, J., Naveh-Many, T. FGF-23 and secondary hyperparathyroidism in chronic kidney disease. Nat Rev Nephrol 9, 641–649 (2013). https://doi.org/10.1038/nrneph.2013.147
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2013.147
This article is cited by
-
Intact fibroblast growth factor 23 in heart failure with reduced and mildly reduced ejection fraction
BMC Cardiovascular Disorders (2023)
-
The Measurement and Interpretation of Fibroblast Growth Factor 23 (FGF23) Concentrations
Calcified Tissue International (2022)
-
Effects of active vitamin D on insulin resistance and islet β-cell function in non-diabetic chronic kidney disease patients: a randomized controlled study
International Urology and Nephrology (2022)
-
Investigation on urinary and serum alpha klotho in dogs with chronic kidney disease
BMC Veterinary Research (2020)
-
FGF23 at the crossroads of phosphate, iron economy and erythropoiesis
Nature Reviews Nephrology (2020)