Abstract
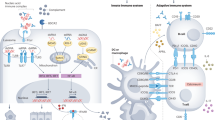
Over the past few years, considerable advances have been made in our understanding of the molecular pathomechanisms of human membranous nephropathy, inspired by studies of Heymann nephritis, a faithful experimental model of this disease. This research led to the identification of neutral endopeptidase, the M-type receptor for secretory phospholipase A2 (PLA2R1) and cationic bovine serum albumin as target antigens of circulating and deposited antibodies in alloimmune neonatal, adult 'idiopathic' and early-childhood membranous nephropathy, respectively. A genome-wide association study has provided further evidence for a highly significant association between PLA2R1 and HLA-DQA1 loci and idiopathic membranous nephropathy in patients of white ancestry. Additional antibody specificities for cytoplasmic antigens have also been identified, but their pathogenic role is uncertain. The time has come to revisit the spectrum of membranous nephropathies based on the newly identified antigen–antibody systems that should be considered as molecular signatures of the disease and that challenge the uniform histological definition. These signatures will soon have a major impact on patient care.
Key Points
-
Antibodies to neutral endopeptidase, the first podocyte antigen described in human membranous nephropathy, are responsible for alloimmune neonatal membranous nephropathy, which develops in children of mothers deficient in this enzyme
-
The secretory phospholipase A2 receptor (PLA2R1) is a major target antigen in idiopathic membranous nephropathy in adults, and antibodies to PLA2R1 are detected in 60–80% of these patients before immunosuppressive treatment
-
In addition to their diagnostic value, anti-PLA2R1 antibodies can be used to monitor response to treatment
-
Variants in PLA2R1 and HLA-DQA1 are strongly associated with idiopathic membranous nephropathy in patients of white ancestry
-
Immunization against cationic bovine serum albumin is a cause of early-childhood membranous nephropathy, which points to a possible role for food and environmental antigens in membranous nephropathy
-
The newly identified antigen–antibody systems should be considered as molecular signatures of membranous nephropathy that challenge the uniform histological definition and will have a major impact on patient care in the near future
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Glassock, R. J. The pathogenesis of idiopathic membranous nephropathy: a 50-year odyssey. Am. J. Kidney Dis. 56, 157–167 (2010).
Kerjaschki, D. Pathogenetic concepts of membranous glomerulopathy (MGN). J. Nephrol. 13 (Suppl. 3), S96–S100 (2000).
Imai, H., Hamai, K., Komatsuda, A., Ohtani, H. & Miura, A. B. IgG subclasses in patients with membranoproliferative glomerulonephritis, membranous nephropathy, and lupus nephritis. Kidney Int. 51, 270–276 (1997).
Kuroki, A. et al. Glomerular and serum IgG subclasses in diffuse proliferative lupus nephritis, membranous lupus nephritis, and idiopathic membranous nephropathy. Intern. Med. 41, 936–942 (2002).
Ohtani, H. et al. Distribution of glomerular IgG subclass deposits in malignancy-associated membranous nephropathy. Nephrol. Dial. Transplant. 19, 574–579 (2004).
Segawa, Y. et al. IgG subclasses and complement pathway in segmental and global membranous nephropathy. Pediatr. Nephrol. 25, 1091–1099 (2010).
Ponticelli, C. Membranous nephropathy. J. Nephrol. 20, 268–287 (2007).
Polanco, N. et al. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J. Am. Soc. Nephrol. 21, 697–704 (2010).
Polanco, N. et al. Spontaneous remission of nephrotic syndrome in membranous nephropathy with chronic renal impairment. Nephrol. Dial. Transplant. 27, 231–234 (2012).
Glassock, R. J. Diagnosis and natural course of membranous nephropathy. Semin. Nephrol. 23, 324–332 (2003).
Cattran, D. Management of membranous nephropathy: when and what for treatment. J. Am. Soc. Nephrol. 16, 1188–1194 (2005).
Glassock, R. J. The treatment of idiopathic membranous nephropathy: a dilemma or a conundrum? Am. J. Kidney Dis. 44, 562–566 (2004).
Waldman, M. & Austin, H. A. III. Controversies in the treatment of idiopathic membranous nephropathy. Nat. Rev. Nephrol. 5, 469–479 (2009).
Cattran, D. C., Reich, H. N., Kim, S. J. & Troyanov, S. Have we changed the outcome in membranous nephropathy? A propensity study on the role of immunosuppressive therapy. Clin. J. Am. Soc. Nephrol. 6, 1591–1598 (2011).
Heymann, W., Hackel, D. B., Harwood, S., Wilson, S. G. & Hunter, J. L. Production of nephrotic syndrome in rats by Freund's adjuvants and rat kidney suspensions. Proc. Soc. Exp. Biol. Med. 100, 660–664 (1959).
Van Damme, B. J., Fleuren, G. J., Bakker, W. W., Vernier, R. L. & Hoedemaeker, P. J. Experimental glomerulonephritis in the rat induced by antibodies directed against tubular antigens. V. Fixed glomerular antigens in the pathogenesis of heterologous immune complex glomerulonephritis. Lab. Invest. 38, 502–510 (1978).
Couser, W. G., Steinmuller, D. R., Stilmant, M. M., Salant, D. J. & Lowenstein, L. M. Experimental glomerulonephritis in the isolated perfused rat kidney. J. Clin. Invest. 62, 1275–1287 (1978).
Border, W. A., Ward, H. J., Kamil, E. S. & Cohen, A. H. Induction of membranous nephropathy in rabbits by administration of an exogenous cationic antigen. J. Clin. Invest. 69, 451–461 (1982).
Adler, S. G., Wang, H., Ward, H. J., Cohen, A. H. & Border, W. A. Electrical charge. Its role in the pathogenesis and prevention of experimental membranous nephropathy in the rabbit. J. Clin. Invest. 71, 487–499 (1983).
Kerjaschki, D. & Farquhar, M. G. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc. Natl Acad. Sci. USA 79, 5557–5561 (1982).
Kerjaschki, D. & Farquhar, M. G. Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J. Exp. Med. 157, 667–686 (1983).
Debiec, H. et al. Early childhood membranous nephropathy due to cationic bovine serum albumin. N. Engl. J. Med. 364, 2101–2110 (2011).
Debiec, H. et al. Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N. Engl. J. Med. 346, 2053–2060 (2002).
Debiec, H. et al. Role of truncating mutations in MME gene in fetomaternal alloimmunisation and antenatal glomerulopathies. Lancet 364, 1252–1259 (2004).
Beck, L. H. Jr et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N. Engl. J. Med. 361, 11–21 (2009).
Stanescu, H. C. et al. Risk HLA-DQA1 and PLA2R1 alleles in idiopathic membranous nephropathy. N. Engl. J. Med. 364, 616–626 (2011).
Prunotto, M. et al. Autoimmunity in membranous nephropathy targets aldose reductase and SOD2. J. Am. Soc. Nephrol. 21, 507–519 (2010).
Bruschi, M. et al. Direct characterization of target podocyte antigens and auto-antibodies in human membranous glomerulonephritis: alfa-enolase and borderline antigens. J. Proteomics 74, 2008–2017 (2011).
Couser, W. G. & Salant, D. J. In situ immune complex formation and glomerular injury. Kidney Int. 17, 1–13 (1980).
Dixon, F. J., Feldman, J. D. & Vazquez, J. J. Experimental glomerulonephritis. The pathogenesis of a laboratory model resembling the spectrum of human glomerulonephritis. J. Exp. Med. 113, 899–920 (1961).
Wilson, C. B. & Dixon, F. J. Quantitation of acute and chronic serum sickness in the rabbit. J. Exp. Med. 134, 7–18 (1971).
Saito, A. et al. Mapping rat megalin: the second cluster of ligand binding repeats contains a 46-amino acid pathogenic epitope involved in the formation of immune deposits in Heymann nephritis. Proc. Natl Acad. Sci. USA 93, 8601–8605 (1996).
Yamazaki, H. et al. All four putative ligand-binding domains in megalin contain pathogenic epitopes capable of inducing passive Heymann nephritis. J. Am. Soc. Nephrol. 9, 1638–1644 (1998).
Allegri, L. et al. Polyvalent antigen-antibody interactions are required for the formation of electron-dense immune deposits in passive Heymann's nephritis. Am. J. Pathol. 125, 1–6 (1986).
Raychowdhury, R., Zheng, G., Brown, D. & McCluskey, R. T. Induction of Heymann nephritis with a gp330/megalin fusion protein. Am. J. Pathol. 148, 1613–1623 (1996).
Oleinikov, A. V., Feliz, B. J. & Makker, S. P. A small N-terminal 60-kD fragment of gp600 (megalin), the major autoantigen of active Heymann nephritis, can induce a full-blown disease. J. Am. Soc. Nephrol. 11, 57–64 (2000).
Tramontano, A. & Makker, S. P. Conformation and glycosylation of a megalin fragment correlate with nephritogenicity in Heymann nephritis. J. Immunol. 172, 2367–2373 (2004).
Tramontano, A., Knight, T., Vizzuso, D. & Makker, S. P. Nested N-terminal megalin fragments induce high-titer autoantibody and attenuated Heymann nephritis. J. Am. Soc. Nephrol. 17, 1979–1985 (2006).
Shah, P., Tramontano, A. & Makker, S. P. Intramolecular epitope spreading in Heymann nephritis. J. Am. Soc. Nephrol. 18, 3060–3066 (2007).
Ronco, P. et al. A monoclonal antibody to brush border and passive Heymann nephritis. Clin. Exp. Immunol. 55, 319–332 (1984).
Assmann, K. J., Tangelder, M. M., Lange, W. P., Tadema, T. M. & Koene, R. A. Membranous glomerulonephritis in the mouse. Kidney Int. 24, 303–312 (1983).
Assmann, K. J. et al. Comparison of antigenic targets involved in antibody-mediated membranous glomerulonephritis in the mouse and rat. Am. J. Pathol. 121, 112–122 (1985).
Assmann, K. J. et al. Involvement of an antigen distinct from the Heymann antigen in membranous glomerulonephritis in the mouse. Lab. Invest. 60, 138–146 (1989).
Meyer, T. N. et al. A new mouse model of immune-mediated podocyte injury. Kidney Int. 72, 841–852 (2007).
Meyer-Schwesinger, C. et al. Nephrotic syndrome and subepithelial deposits in a mouse model of immune-mediated anti-podocyte glomerulonephritis. J. Immunol. 187, 3218–3229 (2011).
Cybulsky, A. V., Quigg, R. J. & Salant, D. J. Experimental membranous nephropathy redux. Am. J. Physiol. Renal Physiol. 289, F660–F671 (2005).
Nangaku, M., Shankland, S. J. & Couser, W. G. Cellular response to injury in membranous nephropathy. J. Am. Soc. Nephrol. 16, 1195–1204 (2005).
Cunningham, P. N. & Quigg, R. J. Contrasting roles of complement activation and its regulation in membranous nephropathy. J. Am. Soc. Nephrol. 16, 1214–1222 (2005).
Koyama, A., Inage, H., Kobayashi, M., Narita, M. & Tojo, S. Effect of chemical cationization of antigen on glomerular localization of immune complexes in active models of serum sickness nephritis in rabbits. Immunology 58, 529–534 (1986).
Bass, P. S., Drake, A. F., Wang, Y., Thomas, J. H. & Davies, D. R. Cationization of bovine serum albumin alters its conformation as well as its charge. Lab. Invest. 62, 185–188 (1990).
Wright, N. G., Mohammed, N. A., Eckersall, P. D. & Nash, A. S. Experimental immune complex glomerulonephritis in dogs receiving cationized bovine serum albumin. Res. Vet. Sci. 38, 322–328 (1985).
Koyama, A. et al. Role of antigenic charge and antibody avidity on the glomerular immune complex localization in serum sickness of mice. Clin. Exp. Immunol. 64, 606–614 (1986).
Chen, J. S. et al. Mouse model of membranous nephropathy induced by cationic bovine serum albumin: antigen dose-response relations and strain differences. Nephrol. Dial. Transplant. 19, 2721–2728 (2004).
Wu, C. C. et al. Kinetics of adaptive immunity to cationic bovine serum albumin-induced membranous nephropathy. Kidney Int. 72, 831–840 (2007).
Urizar, R. E., Cerda, J. & Reilly, A. Glomerulitis induced by cationized bovine serum albumin in the rat. Pediatr. Nephrol. 3, 149–155 (1989).
Schmiedeke, T. M. et al. Histones have high affinity for the glomerular basement membrane. Relevance for immune complex formation in lupus nephritis. J. Exp. Med. 169, 1879–1894 (1989).
Termaat, R. M. et al. Anti-DNA antibodies can bind to the glomerulus via two distinct mechanisms. Kidney Int. 42, 1363–1371 (1992).
Matsuo, S. et al. Experimental glomerulonephritis induced in rats by a lectin and its antibodies. Kidney Int. 36, 1011–1021 (1989).
Iskandar, S. S., Zhang, J. M. & Rodriguez, E. Nephropathy induced in a nephritis-resistant inbred mouse strain with the use of a cationized antigen. Am. J. Pathol. 123, 67–72 (1986).
Ronco, P. & Debiec, H. Molecular pathomechanisms of membranous nephropathy: from Heymann nephritis to alloimmunization. J. Am. Soc. Nephrol. 16, 1205–1213 (2005).
Stahl, R., Hoxha, E. & Fechner, K. PLA2R autoantibodies and recurrent membranous nephropathy after transplantation. N. Engl. J. Med. 363, 496–498 (2010).
Debiec, H. et al. Autoantibodies specific for the phospholipase A2 receptor in recurrent and de novo membranous nephropathy. Am. J. Transplant. 11, 2144–2152 (2011).
East, L. & Isacke, C. M. The mannose receptor family. Biochim. Biophys. Acta 1572, 364–386 (2002).
West, A. P. Jr, Herr, A. B. & Bjorkman, P. J. The chicken yolk sac IgY receptor, a functional equivalent of the mammalian MHC-related Fc receptor, is a phospholipase A2 receptor homolog. Immunity 20, 601–610 (2004).
Llorca, O. Extended and bent conformations of the mannose receptor family. Cell. Mol. Life Sci. 65, 1302–1310 (2008).
Pedchenko, V. et al. Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N. Engl. J. Med. 363, 343–354 (2010).
Pedchenko, V., Vanacore, R. & Hudson, B. Goodpasture's disease: molecular architecture of the autoantigen provides clues to etiology and pathogenesis. Curr. Opin. Nephrol. Hypertens. 20, 290–296 (2011).
Bagchus, W. M., Hoedemaeker, P. J., Vos, J. T. & Bakker, W. W. Thymocytes reacting with heterologous antibodies against GP 330 in autologous immune complex glomerulopathy (AICG) in the rat. The relation between susceptibility for AICG and anti-GP 330-binding thymocytes. Immunobiology 179, 432–444 (1989).
Vaughan, R. W., Demaine, A. G. & Welsh, K. I. A DQA1 allele is strongly associated with idiopathic membranous nephropathy. Tissue Antigens 34, 261–269 (1989).
Dyer, P. A., Short, C. D., Clarke, E. A. & Mallick, N. P. HLA antigen and gene polymorphisms and haplotypes established by family studies in membranous nephropathy. Nephrol. Dial. Transplant. 7 (Suppl. 1), 42–47 (1992).
Berthoux, F. C. et al. Immunogenetics and immunopathology of human primary membranous glomerulonephritis: HLA-A, B, DR antigens; functional activity of splenic macrophage Fc-receptors and peripheral blood T-lymphocyte subpopulations. Clin. Nephrol. 22, 15–20 (1984).
Sacks, S. H., Warner, C., Campbell, R. D. & Dunham, I. Molecular mapping of the HLA class II region in HLA-DR3 associated idiopathic membranous nephropathy. Kidney Int. Suppl. 39, S13–S19 (1993).
Liu, Y. H. et al. Association of phospholipase A2 receptor 1 polymorphisms with idiopathic membranous nephropathy in Chinese patients in Taiwan. J. Biomed. Sci. 17, 81–88 (2010).
Kim, S. et al. Single nucleotide polymorphisms in the phospholipase A2 receptor gene are associated with genetic susceptibility to idiopathic membranous nephropathy. Nephron Clin. Pract. 117, c253–c258 (2011).
Bantis, C. et al. Tumor necrosis factor-α gene G-308A polymorphism is a risk factor for the development of membranous glomerulonephritis. Am. J. Nephrol. 26, 12–15 (2006).
Thibaudin, D. et al. TNFA2 and d2 alleles of the tumor necrosis factor alpha gene polymorphism are associated with onset/occurrence of idiopathic membranous nephropathy. Kidney Int. 71, 431–437 (2007).
Honkanen, E., von Willebrand, E., Teppo, A. M., Törnroth, T. & Grönhagen-Riska, C. Adhesion molecules and urinary tumor necrosis factor-α in idiopathic membranous glomerulonephritis. Kidney Int. 53, 909–917 (1998).
Neale, T. J. et al. Tumor necrosis factor-alpha is expressed by glomerular visceral epithelial cells in human membranous nephropathy. Am. J. Pathol. 146, 1444–1454 (1995).
Tabibzadeh, S. et al. TNF-α induces dyscohesion of epithelial cells. Association with disassembly of actin filaments. Endocrine 3, 549–556 (1995).
Huber, T. B. & Benzing, T. The slit diaphragm: a signaling platform to regulate podocyte function. Curr. Opin. Nephrol. Hypertens. 14, 211–216 (2005).
Lo, W. Y. et al. Association between genetic polymorphisms of the NPHS1 gene and membranous glomerulonephritis in the Taiwanese population. Clin. Chim. Acta 411, 714–718 (2010).
Chen, C. H. et al. Impact of plasminogen activator inhibitor-1 gene polymorphisms on primary membranous nephropathy. Nephrol. Dial. Transplant. 23, 3166–3173 (2008).
Chen, S. Y. et al. Association of STAT4 polymorphisms with susceptibility to primary membranous glomerulonephritis and renal failure. Clin. Chim. Acta 412, 1899–1904 (2011).
Chen, S. Y. et al. Effect of IL-6 C-572G polymorphism on idiopathic membranous nephropathy risk in a Han Chinese population. Ren. Fail. 32, 1172–1176 (2010).
Qin, W. et al. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J. Am. Soc. Nephrol. 22, 1137–1143 (2011).
Knehtl, M. et al. A case of phospholipase A2 receptor-positive membranous nephropathy preceding sarcoid-associated granulomatous tubulointerstitial nephritis. Am. J. Kidney Dis. 57, 140–143 (2011).
Hoxha, E. et al. An immunofluorescence test for phospholipase-A2-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol. Dial. Transplant. 26, 2526–2532 (2011).
Hofstra, J. M., Beck, L. H. Jr, Beck, D. M., Wetzels, J. F. & Salant, D. J. Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin. J. Am. Soc. Nephrol. 6, 1286–1291 (2011).
Beck, L. H. Jr & Salant, D. J. Membranous nephropathy: recent travels and new roads ahead. Kidney Int. 77, 765–770 (2010).
Beck, L. H. Jr et al. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J. Am. Soc. Nephrol. 22, 1543–1550 (2011).
Debiec, H. & Ronco, P. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N. Engl. J. Med. 364, 689–690 (2011).
Zhang, M. et al. Identification of the target self-antigens in reperfusion injury. J. Exp. Med. 203, 141–152 (2006).
Herrera, O. B. et al. A novel pathway of alloantigen presentation by dendritic cells. J. Immunol. 173, 4828–4837 (2004).
Terrier, B. et al. Alpha-enolase: a target of antibodies in infectious and autoimmune diseases. Autoimmun. Rev. 6, 176–182 (2007).
Servettaz, A. et al. Identification of target antigens of antiendothelial cell antibodies in healthy individuals: A proteomic approach. Proteomics 8, 1000–1008 (2008).
Jordan, S. C., Buckingham, B., Sakai, R. & Olson, D. Studies of immune-complex glomerulonephritis mediated by human thyroglobulin. N. Engl. J. Med. 304, 1212–1215 (1981).
Takekoshi, Y. et al. Free “small” and IgG-associated “large” hepatitis B e antigen in the serum and glomerular capillary walls of two patients with membranous glomerulonephritis. N. Engl. J. Med. 300, 814–819 (1979).
Hörl, W. H. & Kerjaschki, D. Membranous glomerulonephritis (MGN). J. Nephrol. 13, 291–316 (2000).
Kain, R. et al. Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat. Med. 14, 1088–1096 (2008).
Lundberg, K. & Venables, P. J. Epitope spreading in animal models: array of hope in rheumatoid arthritis and multiple sclerosis. Arthritis Res. Ther. 10, 122 (2008).
Chen, L. et al. A nephritogenic peptide induces intermolecular epitope spreading on collagen IV in experimental autoimmune glomerulonephritis. J. Am. Soc. Nephrol. 17, 3076–3081 (2006).
Tipping, P. G. & Kitching, A. R. Glomerulonephritis, Th1 and Th2: what's new? Clin. Exp. Immunol. 142, 207–215 (2005).
Kuroki, A., Iyoda, M., Shibata, T. & Sugisaki, T. Th2 cytokines increase and stimulate B cells to produce IgG4 in idiopathic membranous nephropathy. Kidney Int. 68, 302–310 (2005).
van der Zee, J. S., van Swieten, P. & Aalberse, R. C. Inhibition of complement activation by IgG4 antibodies. Clin. Exp. Immunol. 64, 415–422 (1986).
Aalberse, R. C. & Schuurman, J. IgG4 breaking the rules. Immunology 105, 9–19 (2002).
Lhotta, K., Würzner, R. & König, P. Glomerular deposition of mannose-binding lectin in human glomerulonephritis. Nephrol. Dial. Transplant. 14, 881–886 (1999).
Spicer, S. T. et al. Induction of passive Heymann nephritis in complement component 6-deficient PVG rats. J. Immunol. 179, 172–178 (2007).
Leenaerts, P. L., Hall, B. M., Van Damme, B. J., Daha, M. R. & Vanrenterghem, Y. F. Active Heymann nephritis in complement component C6 deficient rats. Kidney Int. 47, 1604–1614 (1995).
Kerjaschki, D. et al. Pathogenic antibodies inhibit the binding of apolipoproteins to megalin/gp330 in passive Heymann nephritis. J. Clin. Invest. 100, 2303–2309 (1997).
Lambeau, G. & Gelb, M. H. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu. Rev. Biochem. 77, 495–520 (2008).
Hanasaki, K. & Arita, H. Phospholipase A2 receptor: a regulator of biological functions of secretory phospholipase A2. Prostaglandins Other Lipid Mediat. 68–69, 71–82 (2002).
Cybulsky, A. V., Takano, T., Papillon, J. & McTavish, A. J. Complement-induced phospholipase A2 activation in experimental membranous nephropathy. Kidney Int. 57, 1052–1062 (2000).
Zvaritch, E., Lambeau, G. & Lazdunski, M. Endocytic properties of the M-type 180-kDa receptor for secretory phospholipases A2 . J. Biol. Chem. 271, 250–257 (1996).
Augert, A. et al. The M-type receptor PLA2R regulates senescence through the p53 pathway. EMBO Rep. 10, 271–277 (2009).
Sis, B. et al. Accelerated expression of senescence associated cell cycle inhibitor p16INK4A in kidneys with glomerular disease. Kidney Int. 71, 218–226 (2007).
Berg, A. L., Nilsson-Ehle, P. & Arnadottir, M. Beneficial effects of ACTH on the serum lipoprotein profile and glomerular function in patients with membranous nephropathy. Kidney Int. 56, 1534–1543 (1999).
Bomback, A. S. et al. Treatment of nephrotic syndrome with adrenocorticotropic hormone (ACTH) gel. Drug Des. Devel. Ther. 5, 147–153 (2011).
Mountjoy, K. G., Robbins, L. S., Mortrud, M. T. & Cone, R. D. The cloning of a family of genes that encode the melanocortin receptors. Science 257, 1248–1251 (1992).
Lindskog, A. et al. Melanocortin 1 receptor agonists reduce proteinuria. J. Am. Soc. Nephrol. 21, 1290–1298 (2010).
Nortier, J. L. et al. Neonatal disease in neutral endopeptidase alloimmunization: lessons for immunological monitoring. Pediatr. Nephrol. 21, 1399–1405 (2006).
Atkinson, C., Qiao, F., Song, H., Gilkeson, G. S. & Tomlinson, S. Low-dose targeted complement inhibition protects against renal disease and other manifestations of autoimmune disease in MRL/lpr mice. J. Immunol. 180, 1231–1238 (2008).
Huang, Y., Qiao, F., Atkinson, C., Holers, V. M. & Tomlinson, S. A novel targeted inhibitor of the alternative pathway of complement and its therapeutic application in ischemia/reperfusion injury. J. Immunol. 181, 8068–8076 (2008).
Acknowledgements
The research of the authors is supported by grants from Agence Nationale de la Recherche (ANR-07-Physio-016–01), and Coordination Theme 1 (Health) of the European Community's Seventh Framework Programme, grant agreement number HEALTH-F2-2007-201,590. The authors thank C. Vial for assistance with language editing.
Author information
Authors and Affiliations
Contributions
P. Ronco and H. Debiec contributed equally to discussion of content for the article, researching data to include in the manuscript, writing, and reviewing and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ronco, P., Debiec, H. Pathogenesis of membranous nephropathy: recent advances and future challenges. Nat Rev Nephrol 8, 203–213 (2012). https://doi.org/10.1038/nrneph.2012.35
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2012.35
This article is cited by
-
Prevalence of neural epidermal growth factor-like 1- and exostosin 1/exostosin 2-associated membranous nephropathy: a single-center retrospective study in Japan
Scientific Reports (2022)
-
Esculentoside A exerts anti-oxidative stress and anti-apoptotic effects in rat experimental membranous nephropathy by regulating MAPK pathway
Molecular & Cellular Toxicology (2022)
-
A cohort study of membranous nephropathy, primary or secondary
BMC Nephrology (2021)
-
Clinicopathological characteristics and prognosis of hepatitis B associated membranous nephropathy and idiopathic membranous nephropathy complicated with hepatitis B virus infection
Scientific Reports (2021)
-
Diagnostic accuracy of anti-phospholipase A2 receptor (PLA2R) antibodies in idiopathic membranous nephropathy: an Italian experience
Journal of Nephrology (2021)