Abstract
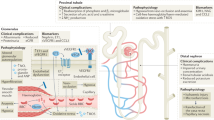
The renal features of sickle cell disease (SCD) include some of the most common reasons for referral to nephrologists, such as hematuria, proteinuria, tubular disturbances and chronic kidney disease. Therapy of these conditions requires specialized knowledge of their distinct pathogenic mechanisms. Painless hematuria is usually benign—unless massive—and can be treated with hydration alone if renal medullary carcinoma has been ruled out. Tubular functional defects, which tend to reduce urinary concentrating capacity, generally require no specific treatment. Proteinuria might indicate the development of chronic sickle cell nephropathy, which can be treated effectively. Measurement of glomerular filtration rate in SCD is problematic, which makes identification and monitoring of chronic kidney disease difficult in patients with SCD. Although managing and predicting the outcomes of chronic kidney disease in the SCD setting is challenging, affected individuals do benefit from transplantation. This Review summarizes the presentation, processes, pathology, modifiers, diagnosis and treatment of the renal effects of SCD.
Key Points
-
Hematuria in sickle cell disease (SCD) or sickle trait can be benign and usually requires only conservative management and an awareness of more-serious potential diagnoses such as renal medullary carcinoma
-
The principal tubule disorder in SCD is defective urinary concentrating capacity, but this complication should not generate serious problems unless there is injudicious use of fluids, NSAIDs or antihypertensive medications, or renal insufficiency
-
Chronic sickle cell nephropathy is a progressive form of focal segmental glomerulosclerosis that seems to begin with hyperperfusion and microalbuminuria and is probably amenable to measures to prevent its progression
-
Transplantation is a good option for patients with advanced sickle cell nephropathy, although the outcomes are not as favorable as for other types of end-stage renal disease
-
The measurement and monitoring of glomerular filtration rate, which is generally elevated in SCD, can be difficult and should be made easier by the use of more refined methods that can correctly detect changes in this parameter
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Osegbe DN (1990) Haematuria and sickle cell disease: a report of 12 cases and review of the literature. Trop Geogr Med 42: 22–27
Oksenhendler E et al. (1984) Recurrent hematuria in 4 white patients with sickle cell trait. J Urol 132: 1201–1203
Pandya KK et al. (1976) Renal papillary necrosis in sickle cell hemoglobinopathies. J Urol 115: 497–501
Odita JC et al. (1983) Urographic changes in homozygous sickle cell disease. Diagn Imaging 52: 259–263
van Eps S and de Jong PE (1988) Sickle cell disease. In Diseases of the Kidney, 2561–2681 (Eds Schrier RW and Gottschalk CW) Boston: Little, Brown and Company
Davidson R and Wilcox CS (1991) Diagnostic usefulness of renal scanning after angiotensin converting enzyme inhibitors. Hypertension 18: 299–303
Burry A et al. (1977) Analgesic nephropathy and the renal concentrating mechanism. Pathol Annu 12: 1–31
Sabatini S (1989) Pathophysiologic mechanisms of abnormal collecting duct function. Semin Nephrol 9: 179–202
Keeler R and Wilson N (1989) Natriuretic response to hypervolemia is absent in rats with papillary necrosis. Am J Physiol 257: R422–R426
Chauhan PM et al. (1983) Pathology of sickle cell disorders. Pathol Annu 18: 253–276
Kersting U et al. (1994) Evidence for an acid pH in rat renal inner medulla: paired measurements with liquid ion-exchange microelectrodes on collecting ducts and vasa recta. Pflugers Arch 426: 354–356
Avery RA et al. (1996) Renal medullary carcinoma: clinical and therapeutic aspects of a newly described tumor. Cancer 78: 128–132
Schultz PK et al. (1991) Hyperechoic renal medullary pyramids in infants and children. Radiology 181: 163–167
Walker TM and Serjeant GR (1995) Increased renal reflectivity in sickle cell disease: prevalence and characteristics. Clin Radiol 50: 566–569
Braden GL et al. (1985) Demeclocycline-induced natriuresis and renal insufficiency: in vivo . Am J Kidney Dis 5: 270–277
McCall IW et al. (1978) Urographic findings in homozygous sickle cell disease. Radiology 126: 99–104
Mapp E et al. (1987) Uroradiological manifestations of S-hemoglobinopathy. Semin Roentgenol 22: 186–194
Lang EK et al. (2004) Multiphasic helical CT diagnosis of early medullary and papillary necrosis. J Endourol 18: 49–56
Brawley OW et al. (2008) National Institutes of Health consensus development conference statement: hydroxyurea treatment for sickle cell disease. Ann Intern Med 148: 932–938
Iyamu EW et al. (2001) Hydroxyurea-induced oxidative damage of normal and sickle cell hemoglobins in vitro: amelioration by radical scavengers. J Clin Lab Anal 15: 1–7
Grindel JM et al. (2002) Distribution, metabolism, and excretion of a novel surface-active agent, purified poloxamer 188, in rats, dogs, and humans. J Pharm Sci 91: 1936–1947
Devereux S and Knowles SM (1985) Rhabdomyolysis and acute renal failure in sickle cell anaemia. Br Med J (Clin Res Ed) 290: 1707
Beutler E (1992) Erythrocyte disorders: anemias related to abnormal globulin. In Hematology, 613–626 (Eds Williams WJ et al.) New York: McGraw-Hill
Weatherall DJ et al. (2001) The hemoglobinopathies. In The Metabolic and Molecular Bases of Inherited Disease, 4571–4626 (Eds Scriver CR et al.) New York: McGraw-Hill
de Jong PE and Statius van Eps LW (1985) Sickle cell nephropathy: new insights into its pathophysiology. Kidney Int 27: 711–717
Allon M (1990) Renal abnormalities in sickle cell disease. Arch Intern Med 150: 501–504
Allon M et al. (1988) Effects of nonsteroidal antiinflammatory drugs on renal function in sickle cell anemia. Kidney Int 34: 500–506
DeFronzo RA et al. (1979) Impaired renal tubular potassium secretion in sickle cell disease. Ann Intern Med 90: 310–316
Herrera J et al. (2002) Impaired creatinine secretion after an intravenous creatinine load is an early characteristic of the nephropathy of sickle cell anaemia. Nephrol Dial Transplant 17: 602–607
Morgan AG et al. (1984) Glomerular function and hyperuricaemia in sickle cell disease. J Clin Pathol 37: 1046–1049
de Jong PE et al. (1983) The influence of indomethacin on renal acidification in normal subjects and in patients with sickle cell anemia. Clin Nephrol 19: 259–264
Buckalew VM and Someren A (1974) Renal manifestations of sickle cell disease. Nephron 133: 660–669
Bakir AA et al. (1987) Prognosis of the nephrotic syndrome in sickle glomerulopathy: a retrospective study. Am J Nephrol 7: 110–115
Powars DR et al. (2005) Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 84: 363–376
Guasch A et al. (2006) Glomerular involvement in adults with sickle cell hemoglobinopathies: prevalence and clinical correlates of progressive renal failure. J Am Soc Nephrol 17: 2228–2235
Sklar AH et al. (1990) A population study of renal function in sickle cell anemia. Int J Artif Organs 13: 231–236
Falk RJ et al. (1992) Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N Engl J Med 326: 910–915
Guasch A et al. (1996) Early detection and the course of glomerular injury in patients with sickle cell anemia. Kidney Int 49: 786–791
Alvarez O et al. (2008) Short-term follow-up of patients with sickle cell disease and albuminuria. Pediatr Blood Cancer 50: 1236–1239
Thompson J et al. (2007) Albuminuria and renal function in homozygous sickle cell disease: observations from a cohort study. Arch Intern Med 167: 701–708
Yoshida Y et al. (1989) Glomerular hemodynamic changes vs hypertrophy in experimental glomerular sclerosis. Kidney Int 35: 654–660
Bhathena DB and Sondheimer JH (1991) The glomerulopathy of homozygous sickle hemoglobin (SS) disease: morphology and pathogenesis. J Am Soc Nephrol 1: 1241–1252
Jennette JC et al. (1987) Glomerulomegaly and focal segmental glomerulosclerosis associated with obesity and sleep-apnea syndrome. Am J Kidney Dis 10: 470–472
Nyberg E et al. (1994) Glomerular volume and renal function in children with different types of the nephrotic syndrome. Pediatr Nephrol 8: 285–289
Spear G (1960) Glomerular alterations in cyanotic congenital heart disease. Nephron 106: 347–367
Olson JL et al. (1982) Altered glomerular permselectivity and progressive sclerosis following extreme ablation of renal mass. Kidney Int 22: 112–126
Foucan L et al. (1998) A randomized trial of captopril for microalbuminuria in normotensive adults with sickle cell anemia. Am J Med 104: 339–342
Woolf AS and Fine LG (1991) Do glomerular hemodynamic adaptations influence the progression of human renal disease? Pediatr Nephrol 5: 88–93
Doi T et al. (1990) Glomerular lesions in mice transgenic for growth hormone and insulinlike growth factor-I: I: relationship between increased glomerular size and mesangial sclerosis. Am J Pathol 137: 541–552
Lande IM et al. (1986) Sickle-cell nephropathy: MR imaging. Radiology 158: 379–383
Guasch A et al. (1999) Evidence that microdeletions in the alpha globin gene protect against the development of sickle cell glomerulopathy in humans. J Am Soc Nephrol 10: 1014–1019
Walser M (1990) Progression of chronic renal failure in man. Kidney Int 37: 1195–1210
Collins AJ et al. (2007) Excerpts from the United States Renal Data System 1996 annual data report. Am J Kidney Dis 49 (Suppl 1): S1–S296
Voskaridou E et al. (2006) markers of renal dysfunction in patients with sickle cell/beta-thalassemia. Kidney Int 69: 2037–2042
Alvarez O et al. (2006) Serum cystatin C levels in children with sickle cell disease. Pediatr Nephrol 21: 533–537
Schwartz GJ et al. (1976) A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263
Cockcroft DW and Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–41
Filler G and Lepage N (2003) Should the Schwartz formula for estimation of GFR be replaced by cystatin C formula? Pediatr Nephrol 18: 981–985
Datta V et al. (2003) Microalbuminuria as a predictor of early glomerular injury in children with sickle cell disease. Indian J Pediatr 70: 307–309
Kattamis A et al. (2004) Clinical response and adverse events in young patients with sickle cell disease treated with hydroxyurea. Pediatr Hematol Oncol 21: 335–342
Tomson CR et al. (1992) Effect of recombinant human erythropoietin on erythropoiesis in homozygous sickle-cell anaemia and renal failure. Nephrol Dial Transplant 7: 817–821
Rodgers GP et al. (1993) Augmentation by erythropoietin of the fetal-hemoglobin response to hydroxyurea in sickle cell disease. N Engl J Med 328: 73–80
Levasseur DN et al. (2003) Correction of a mouse model of sickle cell disease: lentiviral/antisickling beta-globin gene transduction of unmobilized, purified hematopoietic stem cells. Blood 102: 4312–4319
Ojo AO et al. (1999) Renal transplantation in end-stage sickle cell nephropathy. Transplantation 67: 291–295
Bleyer AJ et al. (2001) Relationship between underlying renal disease and renal transplantation outcome. Am J Kidney Dis 37: 1152–1161
Scheinman JI (2004) Sickle cell nephropathy. In Pediatric Nephrology, 917–930 (Eds Avner ED et al.) Philadelphia: Lippincott Williams & Wilkins
Spector D et al. (1978) Painful crises following renal transplantation in sickle cell anemia. Am J Med 64: 835–839
Chatterjee SN (1987) National study in natural history of renal allografts in sickle cell disease or trait: a second report. Transplant Proc 19 (Suppl 2): S33–S35
Donnelly PK et al. (1988) Renal transplantation in sickle cell disease. Lancet 2: 229
Miner DJ et al. (1987) Recurrent sickle cell nephropathy in a transplanted kidney. Am J Kidney Dis 10: 306–313
Smith-Whitley K et al. (2004) Epidemiology of human parvovirus B19 in children with sickle cell disease. Blood 103: 422–427
Johnson CS and Giorgio AJ (1981) Arterial blood pressure in adults with sickle cell disease. Arch Intern Med 141: 891–893
Sklar AH et al. (1990) Acute renal failure in sickle cell anemia. Int J Artif Organs 13: 347–351
Simckes AM et al. (1999) Ketorolac-induced irreversible renal failure in sickle cell disease: a case report. Pediatr Nephrol 13: 63–67
Koppes GM et al. (1977) Exertion-induced rhabdomyolysis with acute renal failure and disseminated intravascular coagulation in sickle cell trait. Am J Med 63: 313–317
Sty JR et al. (1980) Abnormal Tc-99m-methylene diphosphonate accumulation in the kidneys of children with sickle cell disease. Clin Nucl Med 5: 445–447
Swartz MA et al. (2002) Renal medullary carcinoma: clinical, pathologic, immunohistochemical, and genetic analysis with pathogenetic implications. Urology 60: 1083–1089
McCredie M et al. (1986) Phenacetin and papillary necrosis: independent risk factors for renal pelvic cancer. Kidney Int 30: 81–84
Hakimi AA et al. (2007) Renal medullary carcinoma: the Bronx experience. Urology 70: 878–882
Merched AJ et al. (2008) Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J 22: 3595–3606
de Jong PE et al. (1978) The role of prostaglandins and renin in sickle-cell nephropathy: a hypothesis. Neth J Med 21: 67–72
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author has served on an independent renal safety board for Novartis.
Rights and permissions
About this article
Cite this article
Scheinman, J. Sickle cell disease and the kidney. Nat Rev Nephrol 5, 78–88 (2009). https://doi.org/10.1038/ncpneph1008
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpneph1008
This article is cited by
-
Risk factors associated with sickle cell retinopathy: findings from the Cooperative Study of Sickle Cell Disease
International Journal of Retina and Vitreous (2022)
-
Acute kidney injury in hospitalized children with sickle cell anemia
BMC Nephrology (2022)
-
Increasing nitric oxide bioavailability fails to improve collateral vessel formation in humanized sickle cell mice
Laboratory Investigation (2022)
-
Methods to estimate baseline creatinine and define acute kidney injury in lean Ugandan children with severe malaria: a prospective cohort study
BMC Nephrology (2020)
-
Pathophysiology and recent therapeutic insights of sickle cell disease
Annals of Hematology (2020)