Abstract
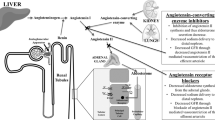
Proteinuria is a major long-term clinical consequence of diabetes and hypertension, conditions that lead to progressive loss of functional renal tissue and, ultimately, end-stage renal disease. Proteinuria is also a strong predictor of cardiovascular events. Convincing preclinical and clinical evidence exists that proteinuria and the underlying glomerulosclerosis are reversible processes. This Review outlines the mechanisms involved in the development of glomerulosclerosis—particularly those responsible for podocyte injury—with an emphasis on the potential capacity of endothelin receptor blockade to reverse this process. There is strong evidence that endothelin-1, a peptide with growth-promoting and vasoconstricting properties, has a central role in the pathogenesis of proteinuria and glomerulosclerosis, which is mediated via activation of the ETA receptor. Several antiproteinuric drugs, including angiotensin-converting-enzyme inhibitors, angiotensin receptor antagonists, statins and certain calcium channel blockers, inhibit the formation of endothelin-1. Preclinical studies have demonstrated that endothelin receptor antagonists can reverse proteinuric renal disease and glomerulosclerosis, and preliminary studies in humans with renal disease have shown that these drugs have remarkable antiproteinuric effects that are additive to those of standard antiproteinuric therapy. Additional clinical studies are needed.
Key Points
-
Podocytes are gatekeepers of the glomerular filtration barrier, and podocyte injury is a prerequisite for development of proteinuria and glomerulosclerosis
-
Endothelin is a potent growth factor and vasoconstrictor, which is highly expressed in the renal vasculature and parenchyma
-
Endothelin disrupts the actin cytoskeleton of podocytes—which is necessary for the structural support and signaling of these cells—and also causes podocyte loss and nephrin shedding
-
Glomerular disease is associated with activation of renal endothelin production and can be prevented or even reversed by endothelin ETA receptor antagonists
-
Preliminary clinical studies indicate that endothelin antagonists can reverse proteinuric renal disease even when superimposed onto standard antiproteinuric therapy
-
Carefully designed, prospective clinical studies of endothelin antagonism in renal disease are required, and should take into account the receptor selectivity and potential toxicity of the drugs and the comorbidities and disease severity of the patients
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Remuzzi G et al. (2006) Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest 116: 288–296
Jaber BL and Madias NE (2005) Progression of chronic kidney disease: can it be prevented or arrested? Am J Med 118: 1323–1330
de Borst MH et al. (2008) Primer: strategies for identifying genes involved in renal disease. Nat Clin Pract Nephrol 4: 265–276
Collins AJ et al. (2005) Excerpts from the United States Renal Data System 2004 annual data report: atlas of end-stage renal disease in the United States. Am J Kidney Dis 45 (1 Suppl 1): A5–A7, S1–S280
Xue JL et al. (2001) Forecast of the number of patients with end-stage renal disease in the United States to the year 2010. J Am Soc Nephrol 12: 2753–2758
Eijkelkamp WB et al. (2007) Albuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy: post hoc analysis from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. J Am Soc Nephrol 18: 1540–1546
Fogo AB (2001) Progression and potential regression of glomerulosclerosis. Kidney Int 59: 804–819
Fogo AB (2005) New capillary growth: a contributor to regression of sclerosis? Curr Opin Nephrol Hypertens 14: 201–203
Fogo AB (2006) Progression versus regression of chronic kidney disease. Nephrol Dial Transplant 21: 281–284
Dussaule JC and Chatziantoniou C (2007) Reversal of renal disease: is it enough to inhibit the action of angiotensin II? Cell Death Differ 14: 1343–1349
Shankland SJ (2006) The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147
Reiser J et al. (2004) Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and alpha3 integrin. J Biol Chem 279: 34827–34832
Floege J et al. (1997) Age-related glomerulosclerosis and interstitial fibrosis in Milan normotensive rats: a podocyte disease. Kidney Int 51: 230–243
Opocensky M et al. (2006) Late-onset endothelin-A receptor blockade reduces podocyte injury in homozygous Ren-2 rats despite severe hypertension. Hypertension 48: 965–971
Nagase M et al. (2006) Podocyte injury underlies the glomerulopathy of Dahl salt-hypertensive rats and is reversed by aldosterone blocker. Hypertension 47: 1084–1093
Shibata S et al. (2006) Fluvastatin ameliorates podocyte injury in proteinuric rats via modulation of excessive Rho signaling. J Am Soc Nephrol 17: 754–764
Shibata S et al. (2007) Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension 49: 355–364
Asanuma K and Mundel P (2003) The role of podocytes in glomerular pathobiology. Clinical Exp Nephrol 7: 255–259
Ziyadeh FN and Wolf G (2008) Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev 4: 39–45
Kalluri R (2006) Proteinuria with and without renal glomerular podocyte effacement. J Am Soc Nephrol 17: 2383–2389
Mahan JD et al. (1986) Glomerular basement membrane anionic charge site changes early in aminonucleoside nephrosis. Am J Pathol 125: 393–401
Rastaldi MP et al. (2006) Glomerular podocytes contain neuron-like functional synaptic vesicles. FASEB J 20: 976–978
Ortmann J et al. (2004) Role of podocytes for reversal of glomerulosclerosis and proteinuria in the aging kidney after endothelin inhibition. Hypertension 44: 974–981
Mundel P (2003) Urinary podocytes: lost and found alive. Kidney Int 64: 1529–1530
Petermann A and Floege J (2007) Podocyte damage resulting in podocyturia: a potential diagnostic marker to assess glomerular disease activity. Nephron Clin Pract 106: c61–c66
Kim YH et al. (2001) Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int 60: 957–968
Macconi D et al. (2006) Permselective dysfunction of podocyte-podocyte contact upon angiotensin II unravels the molecular target for renoprotective intervention. Am J Pathol 168: 1073–1085
Gross ML et al. (2003) ACE-inhibitors but not endothelin receptor blockers prevent podocyte loss in early diabetic nephropathy. Diabetologia 46: 856–868
Gagliardini E et al. (2007) Effect of a selective ETA receptor antagonist on podocyte function and permselective properties of the glomerular barrier in experimental diabetes. Presented at the 10th International Conference on Endothelin: 2007 Bergamo, September 16–19, Bergamo, Italy
Smoyer WE et al. (1997) Podocyte alpha-actinin induction precedes foot process effacement in experimental nephrotic syndrome. Am J Physiol 273: F150–F157
Shankland SJ et al. (2007) Podocytes in culture: past, present, and future. Kidney Int 72: 26–36
Yuan H et al. (2002) Podocyte slit-diaphragm protein nephrin is linked to the actin cytoskeleton. Am J Physiology Renal Physiol 282: F585–F591
Gubler MC (2003) Podocyte differentiation and hereditary proteinuria/nephrotic syndromes. J Am Soc Nephrol 14 (Suppl 1): S22–S26
Endlich N et al. (2001) Podocytes respond to mechanical stress in vitro. J Am Soc Nephrol 12: 413–422
Takeda T et al. (2001) Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest 108: 289–301
Benigni A et al. (2004) Changes in glomerular perm-selectivity induced by angiotensin II imply podocyte dysfunction and slit diaphragm protein rearrangement. Semin Nephrol 24: 131–140
Furchgott RF and Zawadzki JV (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 299: 373–376
Yanagisawa M et al. (1988) A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411–415
Barton M and Yanagisawa M : Endothelin—twenty years from discovery to therapy. Can J Physiol Pharmacol, in press
Rubanyi GM and Polokoff M (1994) Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev 46: 325–415
Ge Y et al. (2006) Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am J Physiol Renal Physiol 291: F1274–F1280
Luscher TF and Barton M (2000) Endothelins and endothelin receptor antagonists: therapeutic considerations for a novel class of cardiovascular drugs. Circulation 102: 2434–2440
Kohan DE (2006) The renal medullary endothelin system in control of sodium and water excretion and systemic blood pressure. Curr Opin Nephrol Hypertens 15: 34–40
Benigni A et al. (1993) A specific endothelin subtype A receptor antagonist protects against injury in renal disease progression. Kidney Int 44: 440–444
Hocher B et al. (1997) Endothelin-1 transgenic mice develop glomerulosclerosis, interstitial fibrosis, and renal cysts but not hypertension. J Clin Invest 99: 1380–1389
Barton M et al. (2000) Dysfunctional renal nitric oxide synthase as a determinant of salt-sensitive hypertension: mechanisms of renal artery endothelial dysfunction and role of endothelin for vascular hypertrophy and glomerulosclerosis. J Am Soc Nephrol 11: 835–845
Vernerová Z et al. (2008) Late-onset endothelin receptor blockade in hypertensive heterozygous REN-2 transgenic rats. Vascul Pharmacol 48: 165–173
Boffa JJ et al. (2001) Regression of renal vascular fibrosis by endothelin receptor antagonism. Hypertension 37: 490–496
Benigni A et al. (1998) Unselective inhibition of endothelin receptors reduces renal dysfunction in experimental diabetes. Diabetes 47: 450–456
Gross ML et al. (2003) Renal damage in the SHR/N-cp type 2 diabetes model: comparison of an angiotensin-converting enzyme inhibitor and endothelin receptor blocker. Lab Invest 83: 1267–1277
Benigni A and Remuzzi G (2001) How renal cytokines and growth factors contribute to renal disease progression. Am J Kidney Dis 37: S21–S24
Gutierrez S et al. (1996) Endothelin-1 induces loss of proteoglycans and enhances fibronectin and collagen production in cultured rabbit synovial cells. Eur J Pharmacol 302: 191–197
Chang JM et al. (2007) Effects of endothelin-1 on thymidine uptake and fibronectin production of diabetic glomeruli. Nephrology (Carlton) 12: 62–66
Morigi M et al. (2006) Shigatoxin-induced endothelin-1 expression in cultured podocytes autocrinally mediates actin remodeling. Am J Pathol 169: 1965–1975
Morigi M et al. (2005) In response to protein load podocytes reorganize cytoskeleton and modulate endothelin-1 gene: implication for permselective dysfunction of chronic nephropathies. Am J Pathol 166: 1309–1320
Nett PC et al. (2006) Recent developments on endothelin antagonists as immunomodulatory drugs—from infection to transplantation medicine. Recent Patents Cardiovasc Drug Discov 1: 265–276
Lattmann T et al. (2005) Activation of pro-inflammatory and anti-inflammatory cytokines in host organs during chronic allograft rejection: role of endothelin receptor signaling. Am J Transplant 5: 1042–1049
Sasser JM et al. (2007) Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J Am Soc Nephrol 18: 143–154
Xia HJ et al. (2006) Up-regulated inflammatory factors endothelin, NFkappaB, TNFalpha and iNOS involved in exaggerated cardiac arrhythmias in l-thyroxine-induced cardiomyopathy are suppressed by darusentan in rats. Life Sci 79: 1812–1819
Gomez-Garre D et al. (1996) An orally active ETA/ETB receptor antagonist ameliorates proteinuria and glomerular lesions in rats with proliferative nephritis. Kidney Int 50: 962–972
Kawaguchi H et al. (1990) Endothelin stimulates angiotensin I to angiotensin II conversion in cultured pulmonary artery endothelial cells. J Mol Cell Cardiol 22: 839–842
Barton M et al. (2000) Obesity is associated with tissue-specific activation of renal angiotensin-converting enzyme in vivo: evidence for a regulatory role of endothelin. Hypertension 35: 329–336
Barton M et al. (1997) Angiotensin II increases vascular and renal endothelin-1 and functional endothelin converting enzyme activity in vivo: role of ETA receptors for endothelin regulation. Biochem Biophys Res Commun 238: 861–865
Rebibou JM et al. (1992) Functional endothelin 1 receptors on human glomerular podocytes and mesangial cells. Nephrol Dial Transplant 7: 288–292
Jia J et al. (2008) Angiotensin II infusion induces nephrin expression changes and podocyte apoptosis. Am J Nephrol 28: 500–507
Zoja C et al. (1999) Protein overload activates proximal tubular cells to release vasoactive and inflammatory mediators. Exp Nephrol 7: 420–428
Wesson DE et al. (2007) Dietary protein induces endothelin-mediated kidney injury through enhanced intrinsic acid production. Kidney Int 71: 210–217
Liang XB et al. (2006) Angiotensin type 1 receptor blocker restores podocyte potential to promote glomerular endothelial cell growth. J Am Soc Nephrol 17: 1886–1895
Benigni A et al. (2004) The potential of endothelin antagonism as a therapeutic approach. Expert Opin Investig Drugs 13: 1419–1435
Gross ML and Amann K (2004) Progression of renal disease: new insights into risk factors and pathomechanisms. Curr Opin Nephrol Hypertens 13: 307–312
Zanatta CM et al. (2008) Endothelin-1 levels and albuminuria in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 80: 299–304
Chen HC et al. (2001) Plasma and urinary endothelin-1 in focal segmental glomerulosclerosis. J Clin Lab Anal 15: 59–63
Susztak K et al. (2006) Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55: 225–233
Zhou X et al. (1995) High glucose alters actin assembly in glomerular mesangial and epithelial cells. Lab Invest 73: 372–383
Petermann AT et al. (2003) Podocytes that detach in experimental membranous nephropathy are viable. Kidney Int 64: 1222–1231
Collino F et al. (2008) Preeclamptic sera induce nephrin shedding from podocytes through endothelin-1 release by endothelial glomerular cells. Am J Physiol. Renal Physiol 294: F1185–F1194
Chen HM et al. (2006) Podocyte lesions in patients with obesity-related glomerulopathy. Am J Kidney Dis 48: 772–779
Saraheimo M et al. (2008) Serum adiponectin and progression of diabetic nephropathy in patients with type 1 diabetes. Diabetes Care 31: 1165–1169
Sharma K et al. (2008) Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest 188: 1645–1656
Barton M et al. (2003) Obesity-associated activation of angiotensin and endothelin in the cardiovascular system. Int J Biochem Cell Biol 35: 826–837
Bedi D et al. (2006) Endothelin-1 inhibits adiponectin secretion through a phosphatidylinositol 4,5-bisphosphate/actin-dependent mechanism. Biochem Biophys Res Commun 345: 332–339
Juan CC et al. (2004) Insulin infusion induces endothelin-1-dependent hypertension in rats. Am J Physiol Endocrinol Metab 287: E948–E954
Yang Z and Li JC (2008) Stimulation of endothelin-1 gene expression by insulin via phosphoinositide-3 kinase-glycogen synthase kinase-3beta signaling in endothelial cells. Life Sci 82: 512–518
Idris I et al. (2001) Tissue- and time-dependent effects of endothelin-1 on insulin-stimulated glucose uptake. Biochem Pharmacol 62: 1705–1708
Aaltonen P et al. (2001) Changes in the expression of nephrin gene and protein in experimental diabetic nephropathy. Lab Invest 81: 1185–1190
Ortmann J et al. (2005) Endothelin inhibition delays onset of hyperglycemia and associated vascular injury in type I diabetes: evidence for endothelin release by pancreatic islet beta-cells. Biochem Biophys Res Commun 334: 689–695
Mauer SM et al. (1974) Pancreatic islet transplantation. Effects on the glomerular lesions of experimental diabetes in the rat. Diabetes 23: 748–753
Lee CS et al. (1974) Renal transplantation in diabetes mellitus in rats. J Exp Med 139: 793–800
Abouna GM et al. (1983) Reversal of diabetic nephropathy in human cadaveric kidneys after transplantation into non-diabetic recipients. Lancet 2: 1274–1276
Fioretto P et al. (1998) Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 339: 69–75
Marinides GN et al. (1990) Enalapril and low protein reverse chronic puromycin aminonucleoside nephropathy. Kidney Int 37: 749–757
Zoja C et al. (1992) Renal protective effect of angiotensin-converting enzyme inhibition in aging rats. Am J Med 92: 60S–63S
Remuzzi A et al. (2006) ACE inhibition reduces glomerulosclerosis and regenerates glomerular tissue in a model of progressive renal disease. Kidney Int 69: 1124–1130
Fujita T et al. (2007) Antiproteinuric effect of the calcium channel blocker cilnidipine added to renin–angiotensin inhibition in hypertensive patients with chronic renal disease. Kidney Int 72: 1543–1549
Ikoma M et al. (1991) Cause of variable therapeutic efficiency of angiotensin converting enzyme inhibitor on glomerular lesions. Kidney Int 40: 195–202
Ostendorf T et al. (1999) VEGF(165) mediates glomerular endothelial repair. J Clin Invest 104: 913–923
Ding SS et al. (2003) Chronic endothelin receptor blockade prevents both early hyperfiltration and late overt diabetic nephropathy in the rat. J Cardiovasc Pharmacol 42: 48–54
Hocher B et al. (2001) Effects of endothelin receptor antagonists on the progression of diabetic nephropathy. Nephron 87: 161–169
Kelly DJ et al. (2000) Effects of endothelin or angiotensin II receptor blockade on diabetes in the transgenic (mRen-2)27 rat. Kidney Int 57: 1882–1894
Schiffrin EL (1999) State-of-the-Art lecture. Role of endothelin-1 in hypertension. Hypertension 34: 876–881
Placier S et al. (2006) Reversal of renal lesions following interruption of nitric oxide synthesis inhibition in transgenic mice. Nephrol Dial Transplant 21: 881–888
Barton M et al. (1998) ETA receptor blockade prevents increased tissue endothelin-1, vascular hypertrophy and endothelial dysfunction in salt-sensitive hypertension. Hypertension 31: 499–504
Honing MLH et al. (2000) ABT-627, a selective ETA-receptor anatagonist, reduces proteinuria in patients with diabetes mellitus. In: Regulation of Vascular Tone in Humans by Endothelium-derived Mediators [thesis] Utrecht: Elinkwijk BV
Wenzel RR et al. (2005) The ETA-selective antagonist SPP301 on top of standard treatment reduces urinary albumin excretion rate in patients with diabetic nephropathy [abstract F-FC093]. Presented at ASN Renal Week: 2005 November 8–13, Philadelphia, PA, USA
Mann. J et al. (2006) Avosentan, a selective endothelin receptor antagonist, decreases albuminuria in patients with diabetic nephropathy. [http://www.associationhq.com/isn/forefronts/pages/ abstractview.php?id=2&page=0] (accessed 5 June 2008)
Viberti G (online 2008) SPP301 (Avosentan) ASCEND clinical results. [http://www.speedel.com/assets/2008_SPP301ASCENDStudy.pdf] (accessed 5 June 2008)
ClinicalTrials.gov (online 2005) [http://clinicaltrials.gov/ct2/show/NCT00120328?term=NCT00120328&rank=1] (accessed 5 June 2008)
Barton M et al. (2006) Role of endothelin receptors for renal protection and survival in hypertension: waiting for clinical trials. Hypertension 48: 834–837
Black HR et al. (2007) Efficacy and safety of darusentan in patients with resistant hypertension: results from a randomized, double-blind, placebo-controlled dose-ranging study. J Clin Hypertens (Greenwich) 9: 760–769
Hernandez-Perera O et al. (1998) Effects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, atorvastatin and simvastatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cells. J Clin Invest 101: 2711–2719
Whaley-Connell A et al. (2008) Attenuation of NADPH oxidase activation and glomerular filtration barrier remodeling with statin treatment. Hypertension 51: 474–480
Eyre J et al. (2007) Statin-sensitive endocytosis of albumin by glomerular podocytes. Am J Physiol Renal Physiol 292: F674–F681
Danesh FR et al. (2002) 3-Hydroxy-3-methylglutaryl CoA reductase inhibitors prevent high glucose-induced proliferation of mesangial cells via modulation of Rho GTPase/p21 signaling pathway: implications for diabetic nephropathy. Proc Natl Acad Sci USA 99: 8301–8305
Whaley-Connell A et al. (2008) Insulin resistance, oxidative stress, and podocyte injury: role of rosuvastatin modulation of filtration barrier injury. Am J Nephrol 28: 67–75
Gianella A et al. (2007) Rosuvastatin treatment prevents progressive kidney inflammation and fibrosis in stroke-prone rats. Am J Pathol 170: 1165–1177
Aldigier JC et al. (2005) Regression of existing glomerulosclerosis by inhibition of aldosterone. J Am Soc Nephrol 16: 3306–3314
Zeng ZP et al. (1992) Endothelin stimulates aldosterone secretion in vitro from normal adrenocortical tissue, but not adenoma tissue, in primary aldosteronism. J Clin Endocrinol Metab 74: 874–878
Rossi GP et al. (1997) Autocrine-paracrine role of endothelin-1 in the regulation of aldosterone synthase expression and intracellular Ca2+ in human adrenocortical carcinoma NCI-H295 cells. Endocrinology 138: 4421–4426
Wang S et al. (2003) Bone morphogenic protein-7 (BMP-7), a novel therapy for diabetic nephropathy. Kidney Int 63: 2037–2049
Zeisberg M et al. (2003) BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 9: 964–968
Kitten AM et al. (1997) Osteogenic protein-1 downregulates endothelin A receptors in primary rat osteoblasts. Am J Physiol 272: E967–E975
De Petris L et al. (2007) Bone morphogenetic protein-7 delays podocyte injury due to high glucose. Nephrol Dial Transplant 22: 3442–3450
Mitu GM et al. (2007) BMP7 is a podocyte survival factor and rescues podocytes from diabetic injury. Am Journal Physiol 293: F1641–F1648
Ueda H et al. (2008) BMP in podocytes is essential for normal glomerular capillary formation. J Am Soc Nephrol 19: 685–694
Parving HH et al. (2008) Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 358: 2433–2446
Benigni A and Remuzzi G (1998) Novel strategies to retard renal disease progression: combining ACE inhibition with endothelin receptor blocking? Nephrol Dial Transplant 13: 2734–2738
Harman C (2007) What new drugs can nephrologists look forward to in the next year or two? Nat Clin Pract Nephrol 3: 235
Orisio S et al. (2007) The SNP5333 gene polymorphism of endothelin A receptor is independently associated with increased albuminuria in type 2 diabetes. Presented at the 10th International Conference on Endothelin: 2007 Bergamo, September 16–19, Bergamo, Italy
Acknowledgements
I thank all present and former collaborators and colleagues who have contributed to the studies discussed in this manuscript, particularly Sidney Shaw, Pierre Moreau, Livius d'Uscio, Philipp Nett, Elvira Haas, Matthias Kretzler, Thomas Lattmann, and Kerstin Amann (who also provided me with the electron microscopy photographs presented in this manuscript). I am indebted to Ariela Benigni for critical reading of the manuscript and many valuable suggestions. I apologize to investigators whose work was not cited because of space limitations. This article was supported by the Swiss National Science Foundation and the University of Zürich. Charles P Vega, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Supplementary information
Supplementary Figure 1
Effect of endothelin blockade on podocyte injury in vivo in a rat model of age-dependent focal segmental glomerulosclerosis. (PDF 97 kb)
Supplementary Figure 2
Effects of endothelin blockade on glomerulosclerosis and proteinuria in vivo in a rat model of age-dependent focal segmental glomerulosclerosis. (PDF 73 kb)
Supplementary Table 1
Summary of clinical and preclinical studies demonstrating functional and/or structural reversal of proteinuric renal disease with various pharmacologic and nonpharmacologic interventions, excluding endothelin receptor antagonists. (DOC 125 kb)
Rights and permissions
About this article
Cite this article
Barton, M. Reversal of proteinuric renal disease and the emerging role of endothelin. Nat Rev Nephrol 4, 490–501 (2008). https://doi.org/10.1038/ncpneph0891
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpneph0891
This article is cited by
-
Antiproteinuric effect of an endothelin-1 receptor antagonist in puromycin aminonucleoside-induced nephrosis in rat
Pediatric Research (2018)
-
Relationship of urinary endothelin-1 with estimated glomerular filtration rate in autosomal dominant polycystic kidney disease: a pilot cross-sectional analysis
BMC Nephrology (2016)
-
Air Pollution-Induced Vascular Dysfunction: Potential Role of Endothelin-1 (ET-1) System
Cardiovascular Toxicology (2016)
-
Endothelin and endothelin antagonists in chronic kidney disease
Kidney International (2014)
-
Disparate effects of single endothelin-A and -B receptor blocker therapy on the progression of renal injury in advanced renovascular disease
Kidney International (2014)