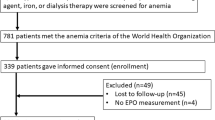
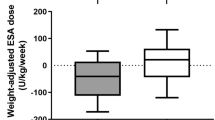
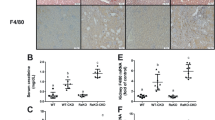
Abstract
Anemia is common in patients who have both heart failure and chronic kidney disease, and there is an association between anemia and progression of both diseases. The main causes of anemia are deficient production of erythropoietin (EPO), iron deficiency, and chronic disease with endogenous EPO resistance. EPO has been successfully used for over a decade to treat anemia in patients with chronic kidney disease. Less obvious are the safety and efficacy of EPO treatment in patients with both heart failure and renal disease. Up to 10% of patients receiving EPO are hyporesponsive to therapy and require large doses of the agent. Several mechanisms could explain resistance to endogenous and exogenous EPO. Proinflammatory cytokines antagonize the action of EPO by exerting an inhibitory effect on erythroid progenitor cells and by disrupting iron metabolism (a process in which hepcidin has a central role). EPO resistance might also be caused by inflammation, which has a negative effect on EPO receptors. Furthermore, neocytolysis could have a role. As resistance to exogenous EPO is associated with an increased risk of death, it is important to understand how cardiorenal failure affects EPO production and function.
Key Points
-
Anemia is common in patients with chronic kidney disease and chronic heart failure, and is associated with a negative outcome
-
Resistance to erythropoietin is a major cause of the anemia that affects patients with both chronic heart failure and chronic kidney disease
-
Inflammation has a key role in resistance to erythropoietin
-
Resistance to exogenous erythropoietin is associated with an increased risk of death in patients with chronic kidney disease
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bongartz LG et al. (2005) The severe cardiorenal syndrome: 'Guyton revisited'. Eur Heart J 26: 11–17
Jie KE et al. (2006) Erythropoietin and the cardiorenal syndrome: cellular mechanisms on the cardiorenal connectors. Am J Physiol Renal Physiol 291: F932–F944
Fehr T et al. (2004) Interpretation of erythropoietin levels in patients with various degrees of renal insufficiency and anemia. Kidney Int 66: 1206–1211
Jacobs C et al. (2003) Results of the European Survey on Anaemia Management 2003 (ESAM 2003): current status of anaemia management in dialysis patients, factors affecting epoetin dosage and changes in anaemia management over the last 5 years. Nephrol Dial Transplant 20 (Suppl 3): Siii3–Siii24
Weiss G and Goodnough LT (2005) Anemia of chronic disease. N Engl J Med 352: 1011–1023
Eckhardt KU (2000) Pathophysiology of renal anemia. Clin Nephrol 53 (Suppl 1): S2–S8
Drueke TB and Eckardt KU (2002) Role of secondary hyperparathyroidism in erythropoietin resistance of chronic renal failure patients. Nephrol Dial Transplant 17 (Suppl 5): S28–S31
de Silva R et al. (2006) Anemia, renal dysfunction, and their interaction in patients with chronic heart failure. Am J Cardiol 98: 391–398
Macdougall IC (1999) The role of ACE inhibitors and angiotensin II receptor blockers in the response to epoetin. Nephrol Dial Transplant 14: 1836–1841
Saudan P et al. (2006) ACE inhibitors or angiotensin II receptor blockers in dialysed patients and erythropoietin resistance. J Nephrol 19: 91–96
Ezekowitz JA et al. (2003) Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12,065 patients with new-onset heart failure. Circulation 107: 223–225
Opasich C et al. (2005) Blunted erythropoietin production and defective iron supply for erythropoiesis as major causes of anaemia in patients with chronic heart failure. Eur Heart J 26: 2232–2237
Anand IS (2005) Pathogenesis of anemia in cardiorenal disease. Rev Cardiovasc Med 6 (Suppl 3): S13–S21
Mrug M et al. (1997) Angiotensin II stimulates proliferation of normal early erythroid progenitors. J Clin Invest 100: 2310–2314
Azizi M et al. (1996) Acute angiotensin-converting enzyme inhibition increases the plasma level of the natural stem cell regulator N-acetyl-seryl-aspartyl-lysyl-proline. J Clin Invest 97: 839–844
van der Meer P et al. (2005) Levels of hematopoiesis inhibitor N-acetyl-seryl-aspartyl-lysyl-proline partially explain the occurrence of anemia in heart failure. Circulation 112: 1743–1747
Albitar S et al. (1998) High dose enalapril impairs the response to erythropoietin treatment in haemodialysis patients. Nephrol Dial Transplant 13: 1206–1210
Erturk S et al. (1999) The impact of withdrawing ACE inhibitors on erythropoietin responsiveness and left ventricular hypertrophy in haemodialysis patients. Nephrol Dial Transplant 14: 1912–1916
Ishani A et al. (2005) Angiotensin-converting enzyme inhibitor as a risk factor for the development of anemia, and the impact of incident anemia on mortality in patients with left ventricular dysfunction. J Am Coll Cardiol 45: 391–399
Androne AS et al. (2003) Hemodilution is common in patients with advanced heart failure. Circulation 107: 226–229
Silverberg DS et al. (2002) The correction of anemia in severe resistant heart failure with erythropoietin and intravenous iron prevents the progression of both the heart and the renal failure and markedly reduces hospitalization. Clin Nephrol 58 (Suppl 1): S37–S45
Levin A et al. (1999) Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis 34: 125–134
Foley RN et al. (1996) The impact of anemia on cardiomyopathy, morbidity, and mortality in end-stage renal disease. Am J Kidney Dis 28: 53–61
Ma JZ et al. (1999) Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol 10: 610–619
Parfrey PS et al. (2005) Double-blind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. J Am Soc Nephrol 16: 2180–2189
Collins AJ et al. (2001) Death, hospitalization, and economic associations among incident hemodialysis patients with hematocrit values of 36 to 39%. J Am Soc Nephrol 12: 2465–2473
Xue JL et al. (2002) Anemia treatment in the pre-ESRD period and associated mortality in elderly patients. Am J Kidney Dis 40: 1153–1161
Locatelli F et al. (2004) Anaemia in haemodialysis patients of five European countries: association with morbidity and mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 19: 121–132
Regidor DL et al. (2006) Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J Am Soc Nephrol 17: 1181–1191
Jones M et al. (2004) Impact of epoetin alfa on clinical end points in patients with chronic renal failure: a meta-analysis. Kidney Int 65: 757–767
Goldberg N et al. (1992) Changes in left ventricular size, wall thickness, and function in anemic patients treated with recombinant human erythropoietin. Am Heart J 124: 424–427
Frank H et al. (2004) Effect of erythropoietin on cardiovascular prognosis parameters in hemodialysis patients. Kidney Int 66: 832–840
Besarab A et al. (1998) The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339: 584–590
Drueke TB et al. for the CREATE Investigators (2006) Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355: 2071–2084
Singh AK et al. for the CHOIR Investigators (2006) Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098
National Kidney Foundation (2006) KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis 47 (Suppl 3): S11–S145
Locatelli F et al. (2004) Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant 19 (Suppl 2): Sii1–Sii47
Anand I et al. (2004) Anemia and its relationship to clinical outcome in heart failure. Circulation 110: 149–154
Go AS et al. (2006) Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation 113: 2713–2723
Mancini DM et al. (2003) Effect of erythropoietin on exercise capacity in patients with moderate to severe chronic heart failure. Circulation 107: 294–299
Silverberg DS et al. (2001) The effect of correction of mild anemia in severe, resistant congestive heart failure using subcutaneous erythropoietin and intravenous iron: a randomized controlled study. J Am Coll Cardiol 37: 1775–1780
Ponikowski P et al. (2007) Effect of darbepoetin alfa on exercise tolerance in anemic patients with symptomatic chronic heart failure: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol 49: 753–762
Palazzuoli A et al. (2006) Erythropoietin improves anemia exercise tolerance and renal function and reduces B-type natriuretic peptide and hospitalization in patients with heart failure and anemia. Am Heart J 152: 1096 e9–e15
Ghali J et al. (2006) Randomized, double-blind, placebo-controlled trial to assess the impact of darbepoetin alfa treatment on exercise tolerance in anemic patients with symptomatic heart failure: results from STAMINA-HeFT (abstract #549). European Society of Cardiology Heart Failure Congress: 2006 17–20 June 2006, Helsinki, Finland
Fisher JW et al. (1996) Erythropoietin production by interstitial cells of hypoxic monkey kidneys. Br J Haematol 95: 27–32
Warnecke C et al. (2004) Differentiating the functional role of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha (EPAS-1) by the use of RNA interference: erythropoietin is a HIF-2alpha target gene in Hep3B and Kelly cells. FASEB J 18: 1462–1464
Scortegagna M et al. (2005) HIF-2alpha regulates murine hematopoietic development in an erythropoietin-dependent manner. Blood 105: 3133–3140
Imagawa S et al. (1997) Negative regulation of the erythropoietin gene expression by the GATA transcription factors. Blood 89: 1430–1439
La Ferla K et al. (2002) Inhibition of erythropoietin gene expression signaling involves the transcription factors GATA-2 and NF-kappaB. FASEB J 16: 1811–1813
Dumitriu B et al. (2006) Sox6 cell-autonomously stimulates erythroid cell survival, proliferation, and terminal maturation and is thereby an important enhancer of definitive erythropoiesis during mouse development. Blood 108: 1198–1207
Fisher JW (2003) Erythropoietin: physiology and pharmacology update. Exp Biol Med (Maywood) 228: 1–14
Wickrema A et al. (1992) Differentiation and erythropoietin receptor gene expression in human erythroid progenitor cells. Blood 80: 1940–1949
Anagnostou A et al. (1994) Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci USA 91: 3974–3978
Chong ZZ et al. (2002) Hematopoietic factor erythropoietin fosters neuroprotection through novel signal transduction cascades. J Cereb Blood Flow Metab 22: 503–514
Henry DH et al. (2004) Epoetin alfa: clinical evolution of a pleiotropic cytokine. Arch Intern Med 164: 262–276
Bahlmann FH et al. (2004) Erythropoietin regulates endothelial progenitor cells. Blood 103: 921–926
Bahlmann FH et al. (2003) Endothelial progenitor cell proliferation and differentiation is regulated by erythropoietin. Kidney Int 64: 1648–1652
George J et al. (2005) Erythropoietin promotes endothelial progenitor cell proliferative and adhesive properties in a PI 3-kinase-dependent manner. Cardiovasc Res 68: 299–306
Beleslin-Cokic BB et al. (2004) Erythropoietin and hypoxia stimulate erythropoietin receptor and nitric oxide production by endothelial cells. Blood 104: 2073–2080
Parsa CJ et al. (2003) A novel protective effect of erythropoietin in the infarcted heart. J Clin Invest 112: 999–1007
Vesey DA et al. (2004) Erythropoietin protects against ischaemic acute renal injury. Nephrol Dial Transplant 19: 348–355
Caro J et al. (1979) Erythropoietin levels in uremic nephric and anephric patients. J Lab Clin Med 93: 449–458
Mason-Garcia M et al. (1990) Development of a new radioimmunoassay for erythropoietin using recombinant erythropoietin. Kidney Int 38: 969–975
Macdougall IC (1995) Poor response to erythropoietin: practical guidelines on investigation and management. Nephrol Dial Transplant 10: 607–614
Volpe M et al. (1994) Blood levels of erythropoietin in congestive heart failure and correlation with clinical, hemodynamic, and hormonal profiles. Am J Cardiol 74: 468–473
Jensen JD et al. (1993) Elevated level of erythropoietin in congestive heart failure relationship to renal perfusion and plasma renin. J Intern Med 233: 125–130
George J et al. (2005) Circulating erythropoietin levels and prognosis in patients with congestive heart failure: comparison with neurohormonal and inflammatory markers. Arch Intern Med 165: 1304–1309
van der Meer P et al. (2004) Prognostic value of plasma erythropoietin on mortality in patients with chronic heart failure. J Am Coll Cardiol 44: 63–67
Stenvinkel P (2001) Inflammatory and atherosclerotic interactions in the depleted uremic patient. Blood Purif 19: 53–61
Bergstrom J et al. (2000) What are the causes and consequences of the chronic inflammatory state in chronic dialysis patients? Semin Dial 13: 163–175
Deswal A et al. (2001) Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation 103: 2055–2059
Levine B et al. (1990) Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 323: 236–241
Dutka DP et al. (1993) Tumour necrosis factor alpha in severe congestive cardiac failure. Br Heart J 70: 141–143
Macdougall IC and Cooper AC (2002) Erythropoietin resistance: the role of inflammation and pro-inflammatory cytokines. Nephrol Dial Transplant 17 (Suppl 11): S39–S43
Means RT Jr (2003) Recent developments in the anemia of chronic disease. Curr Hematol Rep 2: 116–121
Taniguchi S et al. (1997) Interferon gamma downregulates stem cell factor and erythropoietin receptors but not insulin-like growth factor-I receptors in human erythroid colony-forming cells. Blood 90: 2244–2252
Jelkmann W (1998) Proinflammatory cytokines lowering erythropoietin production. J Interferon Cytokine Res 18: 555–559
Maciejewski JP et al. (1995) Nitric oxide suppression of human hematopoiesis in vitro: contribution to inhibitory action of interferon-gamma and tumor necrosis factor-alpha. J Clin Invest 96: 1085–1092
Donovan A et al. (2005) The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab 1: 191–200
Lawrence CM et al. (1999) Crystal structure of the ectodomain of human transferrin receptor. Science 286: 779–782
Nemeth E et al. (2004) IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 113: 1271–1276
Mena NP et al. (2006) Regulation of transepithelial transport of iron by hepcidin. Biol Res 39: 191–193
Dallalio G et al. (2006) Hepcidin inhibits in vitro erythroid colony formation at reduced erythropoietin concentrations. Blood 107: 2702–2704
Malyszko J et al. (2005) Is hepcidin a link between anemia, inflammation and liver function in hemodialyzed patients? Am J Nephrol 25: 586–590
Eleftheriadis T et al. (2006) Does hepcidin affect erythropoiesis in hemodialysis patients? Acta Haematol 116: 238–244
Philo JS et al. (1996) Dimerization of the extracellular domain of the erythropoietin (EPO) receptor by EPO: one high-affinity and one low-affinity interaction. Biochemistry 35: 1681–1691
Constantinescu SN et al. (2001) Ligand-independent oligomerization of cell-surface erythropoietin receptor is mediated by the transmembrane domain. Proc Natl Acad Sci USA 98: 4379–4384
Rossert J and Eckardt KU (2005) Erythropoietin receptors: their role beyond erythropoiesis. Nephrol Dial Transplant 20: 1025–1028
Naranda T et al. (1999) Activation of erythropoietin receptor in the absence of hormone by a peptide that binds to a domain different from the hormone binding site. Proc Natl Acad Sci USA 96: 7569–7574
Wrighton NC et al. (1996) Small peptides as potent mimetics of the protein hormone erythropoietin. Science 273: 458–464
Livnah O et al. (1996) Functional mimicry of a protein hormone by a peptide agonist: the EPO receptor complex at 2.8 A. Science 273: 464–471
Sasaki A et al. (2000) CIS3/SOCS-3 suppresses erythropoietin (EPO) signaling by binding the EPO receptor and JAK2. J Biol Chem 275: 29338–29347
Beckman DL et al. (1999) Activation of the erythropoietin receptor is not required for internalization of bound erythropoietin. Blood 94: 2667–2675
Sorkin A and Waters CM (1993) Endocytosis of growth factor receptors. Bioessays 15: 375–382
Walrafen P et al. (2005) Both proteasomes and lysosomes degrade the activated erythropoietin receptor. Blood 105: 600–608
Witthuhn BA et al. (1993) JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell 74: 227–236
Klingmuller U et al. (1995) Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell 80: 729–738
Akagi S et al. (2004) The critical role of SRC homology domain 2-containing tyrosine phosphatase-1 in recombinant human erythropoietin hyporesponsive anemia in chronic hemodialysis patients. J Am Soc Nephrol 15: 3215–3224
Silva M et al. (1996) Erythropoietin can promote erythroid progenitor survival by repressing apoptosis through Bcl-XL and Bcl-2. Blood 88: 1576–1582
Matsumoto A et al. (1997) CIS, a cytokine inducible SH2 protein, is a target of the JAK-STAT5 pathway and modulates STAT5 activation. Blood 89: 3148–3154
Peltola KJ et al. (2004) Pim-1 kinase inhibits STAT5-dependent transcription via its interactions with SOCS1 and SOCS3. Blood 103: 3744–3750
Miura Y et al. (1994) Activation of the mitogen-activated protein kinase pathway by the erythropoietin receptor. J Biol Chem 269: 29962–29969
Carroll MP et al. (1991) Erythropoietin induces Raf-1 activation and Raf-1 is required for erythropoietin-mediated proliferation. J Biol Chem 266: 14964–14969
Miura O et al. (1994) Erythropoietin-dependent association of phosphatidylinositol 3-kinase with tyrosine-phosphorylated erythropoietin receptor. J Biol Chem 269: 614–620
Kashii Y et al. (2000) A member of Forkhead family transcription factor, FKHRL1, is one of the downstream molecules of phosphatidylinositol 3-kinase-Akt activation pathway in erythropoietin signal transduction. Blood 96: 941–949
Rice L et al. (2001) Neocytolysis on descent from altitude: a newly recognized mechanism for the control of red cell mass. Ann Intern Med 134: 652–656
Trial J and Rice L (2004) Erythropoietin withdrawal leads to the destruction of young red cells at the endothelial-macrophage interface. Curr Pharm Des 10: 183–190
Rice L et al. (1999) Neocytolysis contributes to the anemia of renal disease. Am J Kidney Dis 33: 59–62
Acknowledgements
The EPOCARES study is funded by the Dutch Heart Foundation, Grant #2005B192, The Hague, The Netherlands.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
van der Putten, K., Braam, B., Jie, K. et al. Mechanisms of Disease: erythropoietin resistance in patients with both heart and kidney failure. Nat Rev Nephrol 4, 47–57 (2008). https://doi.org/10.1038/ncpneph0655
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpneph0655
This article is cited by
-
Prevalence, treatment status, and predictors of anemia and erythropoietin hyporesponsiveness in Japanese patients with non-dialysis-dependent chronic kidney disease: a cross-sectional study
Clinical and Experimental Nephrology (2022)
-
Noncardiac comorbidity clustering in heart failure: an overlooked aspect with potential therapeutic door
Heart Failure Reviews (2022)
-
A quantitative systems pharmacology model of hyporesponsiveness to erythropoietin in rats
Journal of Pharmacokinetics and Pharmacodynamics (2021)
-
In search of potential predictors of erythropoiesis-stimulating agents (ESAs) hyporesponsiveness: a population-based study
BMC Nephrology (2019)
-
Achievement of renal anemia KDIGO targets by two different clinical strategies – a European hemodialysis multicenter analysis
BMC Nephrology (2019)