Key Points
-
Circulating alkaline phosphatase (ALP) is a robust and independent predictor of all-cause mortality in the general population and in patients with chronic kidney disease (CKD)
-
Tissue-nonspecific ALP (TNALP) is the most abundant ALP isozyme in the body, comprising >90% of circulating ALP; functional differences between bone ALP (BALP) and liver TNALP are the result of post-translational glycosylation
-
BALP promotes tissue mineralization by inactivating calcification inhibitors and by supplying phosphate
-
Liver ALP and intestinal ALP (IALP) contribute to the immune response through dephosphorylation of circulating endotoxins
-
Modulation of ALP is a potential novel treatment strategy that might reduce vascular calcification and improve cardiovascular outcomes in patients with CKD or diabetes mellitus type 2
Abstract
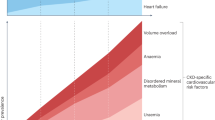
Cardiovascular disease is the main cause of early death in the settings of chronic kidney disease (CKD), type 2 diabetes mellitus (T2DM), and ageing. Cardiovascular events can be caused by an imbalance between promoters and inhibitors of mineralization, which leads to vascular calcification. This process is akin to skeletal mineralization, which is carefully regulated and in which isozymes of alkaline phosphatase (ALP) have a crucial role. Four genes encode ALP isozymes in humans. Intestinal, placental and germ cell ALPs are tissue-specific, whereas the tissue-nonspecific isozyme of ALP (TNALP) is present in several tissues, including bone, liver and kidney. TNALP has a pivotal role in bone calcification. Experimental overexpression of TNALP in the vasculature is sufficient to induce vascular calcification, cardiac hypertrophy and premature death, mimicking the cardiovascular phenotype often found in CKD and T2DM. Intestinal ALP contributes to the gut mucosal defence and intestinal and liver ALPs might contribute to the acute inflammatory response to endogenous or pathogenic stimuli. Here we review novel mechanisms that link ALP to vascular calcification, inflammation, and endothelial dysfunction in kidney and cardiovascular diseases. We also discuss new drugs that target ALP, which have the potential to improve cardiovascular outcomes without inhibiting skeletal mineralization.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Levey, A. S. et al. Chronic kidney disease as a global public health problem: approaches and initiatives — a position statement from kidney disease improving global outcomes. Kidney Int. 72, 247–259 (2007).
Stenvinkel, P. Chronic kidney disease: a public health priority and harbinger of premature cardiovascular disease. J. Intern. Med. 268, 456–467 (2010).
Go, A., Chertow, G., Fan, D., McCulloch, C. & Hsu, C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351, 1296–1305 (2004).
Xie, X. et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta-analysis of randomized clinical trials. Am. J. Kidney Dis. 67, 728–741 (2016).
Palmer, S. C. et al. HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst. Rev. 5, CD007784 (2014).
Stenvinkel, P. & Larsson, T. E. Chronic kidney disease: a clinical model of premature aging. Am. J. Kidney Dis. 62, 339–351 (2013).
Kooman, J. P., Kotanko, P., Schols, A. M., Shiels, P. G. & Stenvinkel, P. Chronic kidney disease and premature ageing. Nat. Rev. Nephrol. 10, 732–742 (2014).
Di Lullo, L. et al. Chronic kidney disease and cardiovascular complications. Heart Fail. Rev. 20, 259–272 (2015).
Li, J. W., Xu, C., Fan, Y., Wang, Y. & Xiao, Y. B. Can serum levels of alkaline phosphatase and phosphate predict cardiovascular diseases and total mortality in individuals with preserved renal function? A systemic review and meta-analysis. PLoS ONE 9, e102276 (2014).
Reid, I. R., Gamble, G. D. & Bolland, M. J. Circulating calcium concentrations, vascular disease and mortality: a systematic review. J. Intern. Med. 279, 524–540 (2016).
Drechsler, C. et al. Bone alkaline phosphatase and mortality in dialysis patients. Clin. J. Am. Soc. Nephrol. 6, 1752–1759 (2011).
Millan, J. L. Mammalian Alkaline Phosphatase: From Biology to Applications in Medicine and Biotechnology (Wiley, 2006).
Magnusson, P., Degerblad, M., Sääf, M., Larsson, L. & Thorén, M. Different responses of bone alkaline phosphatase isoforms during recombinant insulin-like growth factor-i (IGF-I) and during growth hormone therapy in adults with growth hormone deficiency. J. Bone Miner. Res. 12, 210–220 (1997).
Magnusson, P., Löfman, O. & Larsson, L. Determination of alkaline phosphatase isoenzymes in serum by high-performance liquid chromatography with post-column reaction detection. J. Chromatogr. 576, 79–86 (1992).
Magnusson, P. & Farley, J. Differences in sialic acid residues among bone alkaline phosphatase isoforms: a physical, biochemical, and immunological characterization. Calcif. Tissue Int. 71, 508–518 (2002).
Halling Linder, C., Narisawa, S., Millán, J. & Magnusson, P. Glycosylation differences contribute to distinct catalytic properties among bone alkaline phosphatase isoforms. Bone 45, 987–993 (2009).
Weiss, M. J. et al. Structure of the human liver/bone/kidney alkaline phosphatase gene. J. Biol. Chem. 263, 12002–12010 (1988).
Millán, J. L. & Fishman, W. H. Biology of human alkaline phosphatases with special reference to cancer. Crit. Rev. Clin. Lab. Sci. 32, 1–39 (1995).
Magnusson, P. et al. Monoclonal antibodies against tissue-nonspecific alkaline phosphatase. Report of the ISOBM TD9 workshop. Tumour Biol. 23, 228–248 (2002).
Mornet, E. et al. Structural evidence for a functional role of human tissue nonspecific alkaline phosphatase in bone mineralization. J. Biol. Chem. 276, 31171–31178 (2001).
Alcantara, E. H. et al. Zinc deprivation inhibits extracellular matrix calcification through decreased synthesis of matrix proteins in osteoblasts. Mol. Nutr. Food Res. 55, 1552–1560 (2011).
Heaton, F. W. Effect of magnesium deficiency on plasma alkaline phosphatase activity. Nature 207, 1292–1293 (1965).
Hoylaerts, M. F. et al. Functional significance of calcium binding to tissue-nonspecific alkaline phosphatase. PLoS ONE 10, e0119874 (2015).
Fedde, K. N., Lane, C. C. & Whyte, M. P. Alkaline phosphatase is an ectoenzyme that acts on micromolar concentrations of natural substrates at physiologic pH in human osteosarcoma (SAOS-2) cells. Arch. Biochem. Biophys. 264, 400–409 (1988).
Udenfriend, S. & Kodukula, K. How glycosylphosphatidylinositol-anchored membrane proteins are made. Annu. Rev. Biochem. 64, 563–591 (1995).
Low, M. G. The glycosyl-phosphatidylinositol anchor of membrane proteins. Biochim. Biophys. Acta 988, 427–454 (1989).
Hooper, N. M. Glycosyl-phosphatidylinositol anchored membrane enzymes. Clin. Chim. Acta 266, 3–12 (1997).
Magnusson, P., Sharp, C. A. & Farley, J. R. Different distributions of human bone alkaline phosphatase isoforms in serum and bone tissue extracts. Clin. Chim. Acta 325, 59–70 (2002).
Anh, D. J., Eden, A. & Farley, J. R. Quantitation of soluble and skeletal alkaline phosphatase, and insoluble alkaline phosphatase anchor-hydrolase activities in human serum. Clin. Chim. Acta 311, 137–148 (2001).
Magnusson, P., Löfman, O. & Larsson, L. Methodological aspects on separation and reaction conditions of bone and liver alkaline phosphatase isoform analysis by high-performance liquid chromatography. Anal. Biochem. 211, 156–163 (1993).
Magnusson, P. et al. Effect of chronic renal failure on bone turnover and bone alkaline phosphatase isoforms. Kidney Int. 60, 257–265 (2001).
Magnusson, P., Häger, A. & Larsson, L. Serum osteocalcin and bone and liver alkaline phosphatase isoforms in healthy children and adolescents. Pediatr. Res. 38, 955–961 (1995).
Magnusson, P. et al. Differences of bone alkaline phosphatase isoforms in metastatic bone disease and discrepant effects of clodronate on different skeletal sites indicated by the location of pain. Clin. Chem. 44, 1621–1628 (1998).
Magnusson, P., Davie, M. & Sharp, C. Circulating and tissue-derived isoforms of bone alkaline phosphatase in Paget's disease of bone. Scand. J. Clin. Lab. Invest. 70, 128–135 (2010).
Magnusson, P., Larsson, L., Magnusson, M., Davie, M. & Sharp, C. Isoforms of bone alkaline phosphatase: characterization and origin in human trabecular and cortical bone. J. Bone Miner. Res. 14, 1926–1933 (1999).
Swolin-Eide, D., Hansson, S., Larsson, L. & Magnusson, P. The novel bone alkaline phosphatase b1x isoform in children with kidney disease. Pediatr. Nephrol. 21, 1723–1729 (2006).
Haarhaus, M., Fernström, A., Magnusson, M. & Magnusson, P. Clinical significance of bone alkaline phosphatase isoforms, including the novel b1x isoform, in mild to moderate chronic kidney disease. Nephrol. Dial. Transplant. 24, 3382–3389 (2009).
Haarhaus, M., Monier-Faugere, M. C., Magnusson, P. & Malluche, H. H. Bone alkaline phosphatase isoforms in hemodialysis patients with low versus non-low bone turnover: a diagnostic test study. Am. J. Kidney Dis. 66, 99–105 (2015).
Shioi, A. et al. Induction of bone-type alkaline phosphatase in human vascular smooth muscle cells: roles of tumor necrosis factor-alpha and oncostatin M derived from macrophages. Circ. Res. 91, 9–16 (2002).
Haarhaus, M., Arnqvist, H. J. & Magnusson, P. Calcifying human aortic smooth muscle cells express different bone alkaline phosphatase isoforms, including the novel b1x isoform. J. Vasc. Res. 50, 167–174 (2013).
Grabowski, P. Physiology of bone. Endocr. Dev. 28, 33–55 (2015).
Bleicher, F. Odontoblast physiology. Exp. Cell Res. 325, 65–71 (2014).
Golub, E. E. Biomineralization and matrix vesicles in biology and pathology. Semin. Immunopathol. 33, 409–417 (2011).
New, S. E. et al. Macrophage-derived matrix vesicles: an alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ. Res. 113, 72–77 (2013).
Allahverdian, S., Chehroudi, A. C., McManus, B. M., Abraham, T. & Francis, G. A. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 129, 1551–1559 (2014).
Kramann, R. et al. Adventitial msc-like cells are progenitors of vascular smooth muscle cells and drive vascular calcification in chronic kidney disease. Cell Stem Cell 19, 628–642 (2016).
Chen, N., O'Neill, K., Chen, X. & Moe, S. Annexin-mediated matrix vesicle calcification in vascular smooth muscle cells. J. Bone Miner. Res. 23, 1798–1805 (2008).
Millan, J. L. The role of phosphatases in the initiation of skeletal mineralization. Calcif. Tissue Int. 93, 299–306 (2013).
Ali, S. Y., Sajdera, S. W. & Anderson, H. C. Isolation and characterization of calcifying matrix vesicles from epiphyseal cartilage. Proc. Natl Acad. Sci. USA 67, 1513–1520 (1970).
Dai, X. Y. et al. Phosphate-induced autophagy counteracts vascular calcification by reducing matrix vesicle release. Kidney Int. 83, 1042–1051 (2013).
Goettsch, C. et al. Sortilin mediates vascular calcification via its recruitment into extracellular vesicles. J. Clin. Invest. 126, 1323–1336 (2016).
Fleish, H. & Neuman, W. F. Mechanisms of calcification: role of collagen, polyphosphates, and phosphatase. Am. J. Physiol. 200, 1296–1300 (1961).
Fleisch, H. & Bisaz, S. Mechanism of calcification: inhibitory role of pyrophosphate. Nature 195, 911 (1962).
Hessle, L. et al. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc. Natl Acad. Sci. USA 99, 9445–9449 (2002).
Jono, S., Peinado, C. & Giachelli, C. Phosphorylation of osteopontin is required for inhibition of vascular smooth muscle cell calcification. J. Biol. Chem. 275, 20197–20203 (2000).
Halling Linder, C. et al. Bone alkaline phosphatase and tartrate-resistant acid phosphatase: potential co-regulators of bone mineralization. Calcif. Tissue Int. http://dx.doi.org/10.1007/s00223-017-0259-2 (2017).
Robison, R. The possible significance of hexosephosphoric esters in ossification. Biochem. J. 17, 286–293 (1923).
Villa-Bellosta, R. & Egido, J. Phosphate, pyrophosphate, and vascular calcification: a question of balance. Eur. Heart J. http://dx.doi.org/10.1093/eurheartj/ehv605 (2015).
Lomashvili, K. A., Narisawa, S., Millan, J. L. & O'Neill, W. C. Vascular calcification is dependent on plasma levels of pyrophosphate. Kidney Int. 85, 1351–1356 (2014).
de Oliveira, R. A. et al. Peritoneal dialysis per se is a risk factor for sclerostin-associated adynamic bone disease. Kidney Int. 87, 1039–1045 (2015).
Murali, S. K. et al. Fgf23 regulates bone mineralization in a 1,25(OH)2D3 and Klotho-independent manner. J. Bone Miner. Res. 31, 129–142 (2016).
Lieben, L. et al. Normocalcemia is maintained in mice under conditions of calcium malabsorption by vitamin D-induced inhibition of bone mineralization. J. Clin. Invest. 122, 1803–1815 (2012).
Villa-Bellosta, R., Wang, X., Millan, J. L., Dubyak, G. R. & O'Neill, W. C. Extracellular pyrophosphate metabolism and calcification in vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 301, H61–H68 (2011).
Moe, S. et al. Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int. 61, 638–647 (2002).
Shroff, R. et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation 118, 1748–1757 (2008).
Shanahan, C. et al. Medial localization of mineralization-regulating proteins in association with mönckeberg's sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation 100, 2168–2176 (1999).
Chen, N. & Moe, S. Arterial calcification in diabetes. Curr. Diab. Rep. 3, 28–32 (2003).
St Hilaire, C. et al. Nt5e mutations and arterial calcifications. N. Engl. J. Med. 364, 432–442 (2011).
Villa-Bellosta, R. et al. Defective extracellular pyrophosphate metabolism promotes vascular calcification in a mouse model of Hutchinson–Gilford progeria syndrome that is ameliorated on pyrophosphate treatment. Circulation 127, 2442–2451 (2013).
Sheen, C. R. et al. Pathophysiological role of vascular smooth muscle alkaline phosphatase in medial artery calcification. J. Bone Miner. Res. 30, 824–836 (2015).
Savinov, A. Y. et al. Transgenic overexpression of tissue-nonspecific alkaline phosphatase (TNAP) in vascular endothelium results in generalized arterial calcification. J. Am. Heart Assoc. 4, e002499 (2015).
Chen, N. X. & Moe, S. M. Pathophysiology of vascular calcification. Curr. Osteoporos. Rep. 13, 372–380 (2015).
Graciolli, F. et al. Phosphorus overload and PTH induce aortic expression of Runx2 in experimental uraemia. Nephrol. Dial. Transplant. 24, 1416–1421 (2009).
Tangri, N. et al. Effect of bone mineral guideline target achievement on mortality in incident dialysis patients: an analysis of the United Kingdom Renal Registry. Am. J. Kidney Dis. 57, 415–421 (2011).
Sardiwal, S., Magnusson, P., Goldsmith, D. J. & Lamb, E. J. Bone alkaline phosphatase in CKD-mineral bone disorder. Am. J. Kidney Dis. 62, 810–822 (2013).
Palmer, S. C. et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA 305, 1119–1127 (2011).
Fahrleitner-Pammer, A. et al. Bone markers predict cardiovascular events in chronic kidney disease. J. Bone Miner. Res. 23, 1850–1858 (2008).
Damera, S. et al. Serum alkaline phosphatase levels associate with elevated serum c-reactive protein in chronic kidney disease. Kidney Int. 79, 228–233 (2011).
Nayeem, F., Anderson, K. E., Nagamani, M., Grady, J. J. & Lu, L. J. Alkaline phosphatase and percentage body fat predict circulating c-reactive protein in premenopausal women. Biomarkers 15, 663–670 (2010).
Lee, C. T. et al. Association between c-reactive protein and biomarkers of bone and mineral metabolism in chronic hemodialysis patients: a cross-sectional study. J. Ren. Nutr. 19, 220–227 (2009).
Cheung, B. M. et al. Association between plasma alkaline phosphatase and c-reactive protein in Hong Kong Chinese. Clin. Chem. Lab. Med. 46, 523–527 (2008).
Kunutsor, S. K. et al. Serum alkaline phosphatase and risk of incident cardiovascular disease: interrelationship with high sensitivity c-reactive protein. PLoS ONE 10, e0132822 (2015).
Filipowicz, R. et al. Associations of serum skeletal alkaline phosphatase with elevated c-reactive protein and mortality. Clin. J. Am. Soc. Nephrol. 8, 26–32 (2013).
Wannamethee, S. G., Sattar, N., Papcosta, O., Lennon, L. & Whincup, P. H. Alkaline phosphatase, serum phosphate, and incident cardiovascular disease and total mortality in older men. Arterioscler. Thromb. Vasc. Biol. 33, 1070–1076 (2013).
Cho, I. J. et al. Effects of c-reactive protein on bone cells. Life Sci. 145, 1–8 (2016).
Lencel, P., Delplace, S., Hardouin, P. & Magne, D. TNF-α stimulates alkaline phosphatase and mineralization through PPARγ inhibition in human osteoblasts. Bone 48, 242–249 (2011).
Aghagolzadeh, P. et al. Calcification of vascular smooth muscle cells is induced by secondary calciprotein particles and enhanced by tumor necrosis factor-alpha. Atherosclerosis 251, 404–414 (2016).
Shanmugham, L. N. et al. Il-1beta induces alkaline phosphatase in human phagocytes. Arch. Med. Res. 38, 39–44 (2007).
Kukulski, F., Levesque, S. A. & Sevigny, J. Impact of ectoenzymes on p2 and p1 receptor signaling. Adv. Pharmacol. 61, 263–299 (2011).
Antonioli, L., Pacher, P., Vizi, E. S. & Hasko, G. Cd39 and cd73 in immunity and inflammation. Trends Mol. Med. 19, 355–367 (2013).
Fawley, J. & Gourlay, D. M. Intestinal alkaline phosphatase: a summary of its role in clinical disease. J. Surg. Res. 202, 225–234 (2016).
Okada, T., Zinchuk, V. S. & Seguchi, H. Lipopolysaccharide administration increases acid and alkaline phosphatase reactivity in the cardiac muscle. Microsc. Res. Tech. 58, 421–426 (2002).
Pike, A. F., Kramer, N. I., Blaauboer, B. J., Seinen, W. & Brands, R. A novel hypothesis for an alkaline phosphatase 'rescue' mechanism in the hepatic acute phase immune response. Biochim. Biophys. Acta 1832, 2044–2056 (2013).
Jalkanen, J. et al. Aberrant circulating levels of purinergic signaling markers are associated with several key aspects of peripheral atherosclerosis and thrombosis. Circ. Res. 116, 1206–1215 (2015).
Qian, S. et al. The P2Y2 nucleotide receptor is an inhibitor of vascular calcification. Atherosclerosis 257, 38–46 (2017).
Lalles, J. P. Intestinal alkaline phosphatase: novel functions and protective effects. Nutr. Rev. 72, 82–94 (2014).
Alpers, D. H. et al. Intestinal alkaline phosphatase in patients with chronic renal failure. Gastroenterology 94, 62–67 (1988).
De Broe, M. E., Bosteels, V. & Wieme, R. J. Letter: increased intestinal alkaline phosphatase in serum of patients on maintenance haemodialysis. Lancet 303, 753–754 (1974).
Stepan, J., Pilarova, T. & Melicharova, D. The source and clinical significance of serum alkaline phosphatases in patients treated by chronic dialysis. Acta Univ. Carol. Med. Monogr. 78, 75–79 (1977).
Tibi, L., Chhabra, S. C., Sweeting, V. M., Winney, R. J. & Smith, A. F. Multiple forms of alkaline phosphatase in plasma of hemodialysis patients. Clin. Chem. 37, 815–820 (1991).
Zetterberg, H. Increased serum concentrations of intestinal alkaline phosphatase in peritoneal dialysis. Clin. Chem. 51, 675–676 (2005).
Raj, D. S. et al. Soluble CD14 levels, interleukin 6, and mortality among prevalent hemodialysis patients. Am. J. Kidney Dis. 54, 1072–1080 (2009).
Andersen, K. et al. Intestinal dysbiosis, barrier dysfunction, and bacterial translocation account for CKD-related systemic inflammation. J. Am. Soc. Nephrol. 28, 76–83 (2016).
Kieffer, D. A. et al. Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats. Am. J. Physiol. Renal Physiol. 310, F857–F871 (2016).
Malo, M. S. et al. Intestinal alkaline phosphatase preserves the normal homeostasis of gut microbiota. Gut 59, 1476–1484 (2010).
Aronsson, B., Barany, P., Nord, C. E., Nystrom, B. & Stenvinkel, P. Clostridium difficile-associated diarrhoea in uremic patients. Eur. J. Clin. Microbiol. 6, 352–356 (1987).
Alam, S. N. et al. Intestinal alkaline phosphatase prevents antibiotic-induced susceptibility to enteric pathogens. Ann. Surg. 259, 715–722 (2014).
Singh, A. K. & Kari, J. A. Metabolic syndrome and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 22, 198–203 (2013).
Kaliannan, K. et al. Intestinal alkaline phosphatase prevents metabolic syndrome in mice. Proc. Natl Acad. Sci. USA 110, 7003–7008 (2013).
Malo, M. S. A high level of intestinal alkaline phosphatase is protective against type 2 diabetes mellitus irrespective of obesity. EBioMedicine 2, 2016–2023 (2015).
Schultz-Hector, S., Balz, K., Bohm, M., Ikehara, Y. & Rieke, L. Cellular localization of endothelial alkaline phosphatase reaction product and enzyme protein in the myocardium. J. Histochem. Cytochem. 41, 1813–1821 (1993).
Deracinois, B., Lenfant, A. M., Dehouck, M. P. & Flahaut, C. Tissue non-specific alkaline phosphatase (TNAP) in vessels of the brain. Subcell. Biochem. 76, 125–151 (2015).
Liu, Y., Drozdov, I., Shroff, R., Beltran, L. E. & Shanahan, C. M. Prelamin A accelerates vascular calcification via activation of the DNA damage response and senescence-associated secretory phenotype in vascular smooth muscle cells. Circ. Res. 112, e99–e109 (2013).
Yamazaki, Y. et al. Vascular cell senescence contributes to blood-brain barrier breakdown. Stroke 47, 1068–1077 (2016).
Pike, A. F., Kramer, N. I., Blaauboer, B. J., Seinen, W. & Brands, R. An alkaline phosphatase transport mechanism in the pathogenesis of Alzheimer's disease and neurodegeneration. Chem. Biol. Interact. 226, 30–39 (2015).
Perticone, F. et al. Serum alkaline phosphatase negatively affects endothelium-dependent vasodilation in naive hypertensive patients. Hypertension 66, 874–880 (2015).
Mody, N., Parhami, F., Sarafian, T. & Demer, L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic. Biol. Med. 31, 509–519 (2001).
Muteliefu, G., Enomoto, A., Jiang, P., Takahashi, M. & Niwa, T. Indoxyl sulphate induces oxidative stress and the expression of osteoblast-specific proteins in vascular smooth muscle cells. Nephrol. Dial. Transplant. 24, 2051–2058 (2009).
Watanabe, H. et al. P-cresyl sulfate, a uremic toxin, causes vascular endothelial and smooth muscle cell damages by inducing oxidative stress. Pharmacol. Res. Perspect. 3, e00092 (2015).
Raghuraman, G. et al. Eotaxin augments calcification in vascular smooth muscle cells. J. Cell. Biochem. 118, 647–654 (2017).
Torino, C. et al. Oxidative stress as estimated by gamma-glutamyl transferase levels amplifies the alkaline phosphatase-dependent risk for mortality in ESKD patients on dialysis. Oxid. Med. Cell. Longev. 2016, 8490643 (2016).
Oh, S. W., Han, K. H. & Han, S. Y. Associations between renal hyperfiltration and serum alkaline phosphatase. PLoS ONE 10, e0122921 (2015).
Bulum, T., Kolaric, B., Duvnjak, M. & Duvnjak, L. Alkaline phosphatase is independently associated with renal function in normoalbuminuric type 1 diabetic patients. Ren. Fail. 36, 372–377 (2014).
Pfleiderer, G. et al. Change in alkaline phosphatase isoenzyme pattern in urine as possible marker for renal disease. Kidney Int. 17, 242–249 (1980).
Tobar, A. et al. Proximal tubular hypertrophy and enlarged glomerular and proximal tubular urinary space in obese subjects with proteinuria. PLoS ONE 8, e75547 (2013).
Canales, B. K. et al. Renal glomerular and tubular injury after gastric bypass in obese rats. Nutrition 28, 76–80 (2012).
Kapojos, J. J. et al. Induction of glomerular alkaline phosphatase after challenge with lipopolysaccharide. Int. J. Exp. Pathol. 84, 135–144 (2003).
Briere, N., Petitclerc, C. & Plante, G. Presence of alkaline phosphatase and gamma-glutamyl transpeptidase on the parietal layer of Bowman's capsule. Acta Histochem. 73, 237–241 (1983).
Briere, N., Plante, G. E. & Petitclerc, C. Electron-microscopic demonstration of alkaline-phosphatase activity in the juxtaglomerular apparatus. Cell. Tissue Res. 234, 147–151 (1983).
Whyte, M. P. Hypophosphatasia — aetiology, nosology, pathogenesis, diagnosis and treatment. Nat. Rev. Endocrinol. 12, 233–246 (2016).
Kunutsor, S. K., Apekey, T. A. & Khan, H. Liver enzymes and risk of cardiovascular disease in the general population: a meta-analysis of prospective cohort studies. Atherosclerosis 236, 7–17 (2014).
Iqbal, M. P., Mehboobali, N., Azam, I. & Tareen, A. K. Association of alkaline phosphatase with acute myocardial infarction in a population with high prevalence of hypovitaminosis D. Clin. Chim. Acta 425, 192–195 (2013).
Park, J. B. et al. Serum alkaline phosphatase is a predictor of mortality, myocardial infarction, or stent thrombosis after implantation of coronary drug-eluting stent. Eur. Heart J. 34, 920–931 (2013).
Shantouf, R. et al. Association of serum alkaline phosphatase with coronary artery calcification in maintenance hemodialysis patients. Clin. J. Am. Soc. Nephrol. 4, 1106–1114 (2009).
London, G. et al. Arterial calcifications and bone histomorphometry in end-stage renal disease. J. Am. Soc. Nephrol. 15, 1943–1951 (2004).
Barreto, D. V. et al. Association of changes in bone remodeling and coronary calcification in hemodialysis patients: a prospective study. Am. J. Kidney Dis. 52, 1139–1150 (2008).
Kurz, P. et al. Evidence for abnormal calcium homeostasis in patients with adynamic bone disease. Kidney Int. 46, 855–861 (1994).
Bover, J. et al. Adynamic bone disease: from bone to vessels in chronic kidney disease. Semin. Nephrol. 34, 626–640 (2014).
Briet, M. et al. Age-independent association between arterial and bone remodeling in mild-to-moderate chronic kidney disease. Nephrol. Dial. Transplant. 25, 191–197 (2010).
Lomashvili, K., Garg, P., Narisawa, S., Millan, J. & O'Neill, W. Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: potential mechanism for uremic vascular calcification. Kidney Int. 73, 1024–1030 (2008).
Lomashvili, K., Khawandi, W. & O'Neill, W. Reduced plasma pyrophosphate levels in hemodialysis patients. J. Am. Soc. Nephrol. 16, 2495–2500 (2005).
O'Neill, W. C., Lomashvili, K. A., Malluche, H. H., Faugere, M. C. & Riser, B. L. Treatment with pyrophosphate inhibits uremic vascular calcification. Kidney Int. 79, 512–517 (2011).
Tonelli, M. et al. Relation between alkaline phosphatase, serum phosphate, and all-cause or cardiovascular mortality. Circulation 120, 1784–1792 (2009).
Kalantar-Zadeh, K. et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 70, 771–780 (2006).
Regidor, D. et al. Serum alkaline phosphatase predicts mortality among maintenance hemodialysis patients. J. Am. Soc. Nephrol. 19, 2193–2203 (2008).
Blayney, M. J. et al. High alkaline phosphatase levels in hemodialysis patients are associated with higher risk of hospitalization and death. Kidney Int. 74, 655–663 (2008).
Beddhu, S., Ma, X., Baird, B., Cheung, A. & Greene, T. Serum alkaline phosphatase and mortality in African Americans with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 4, 1805–1810 (2009).
Kovesdy, C. P., Ureche, V., Lu, J. L. & Kalantar-Zadeh, K. Outcome predictability of serum alkaline phosphatase in men with pre-dialysis CKD. Nephrol. Dial. Transplant. 25, 3003–3011 (2010).
Beddhu, S., Baird, B., Ma, X., Cheung, A. K. & Greene, T. Serum alkaline phosphatase and mortality in hemodialysis patients. Clin. Nephrol. 74, 91–96 (2010).
Lukowsky, L. R. et al. Mineral and bone disorders and survival in hemodialysis patients with and without polycystic kidney disease. Nephrol. Dial. Transplant. 27, 2899–2907 (2012).
Rhee, C. M. et al. Comparative mortality-predictability using alkaline phosphatase and parathyroid hormone in patients on peritoneal dialysis and hemodialysis. Perit. Dial. Int. 34, 732–748 (2014).
Liu, X. et al. Alkaline phosphatase and mortality in patients on peritoneal dialysis. Clin. J. Am. Soc. Nephrol. 9, 771–778 (2014).
Maruyama, Y. et al. A higher serum alkaline phosphatase is associated with the incidence of hip fracture and mortality among patients receiving hemodialysis in Japan. Nephrol. Dial. Transplant. 29, 1532–1538 (2014).
Fein, P. A. et al. Relationship between alkaline phosphatase and all-cause mortality in peritoneal dialysis patients. Adv. Perit. Dial. 29, 61–63 (2013).
Lertdumrongluk, P. et al. Impact of age on survival predictability of bone turnover markers in hemodialysis patients. Nephrol. Dial. Transplant. 28, 2535–2545 (2013).
Taliercio, J. J. et al. Prognostic importance of serum alkaline phosphatase in CKD stages 3–4 in a clinical population. Am. J. Kidney Dis. 62, 703–710 (2013).
Beige, J. et al. Association of serum alkaline phosphatase with mortality in non-selected European patients with CKD5D: an observational, three-centre survival analysis. BMJ Open 4, e004275 (2014).
Soohoo, M. et al. Changes in markers of mineral and bone disorders and mortality in incident hemodialysis patients. Am. J. Nephrol. 43, 85–96 (2016).
Molnar, M. Z., Kovesdy, C. P., Mucsi, I., Salusky, I. B. & Kalantar-Zadeh, K. Association of pre-kidney transplant markers of mineral and bone disorder with post-transplant outcomes. Clin. J. Am. Soc. Nephrol. 7, 1859–1871 (2012).
Zelle, D. M. et al. Markers of the hepatic component of the metabolic syndrome as predictors of mortality in renal transplant recipients. Am. J. Transplant. 10, 106–114 (2010).
Foley, R. N. et al. Hypocalcemia, morbidity, and mortality in end-stage renal disease. Am. J. Nephrol. 16, 386–393 (1996).
Chang, J. F. et al. Combined alkaline phosphatase and phosphorus levels as a predictor of mortality in maintenance hemodialysis patients. Medicine (Baltimore) 93, e106 (2014).
Chua, H. R. et al. Predicting first-year mortality in incident dialysis patients with end-stage renal disease — the UREA5 study. Blood Purif. 37, 85–92 (2014).
Lin, Y. C. et al. Effect modifying role of serum calcium on mortality-predictability of PTH and alkaline phosphatase in hemodialysis patients: an investigation using data from the Taiwan Renal Registry Data System from 2005 to 2012. PLoS ONE 10, e0129737 (2015).
Zhu, J. G. et al. Serum alkaline phosphatase levels are not associated with increased death risk in prevalent hemodialysis patients: 5-year experience in a single hemodialysis center. Kidney Blood Press. Res. 41, 498–506 (2016).
Scialla, J. J. et al. Race, mineral homeostasis and mortality in patients with end-stage renal disease on dialysis. Am. J. Nephrol. 42, 25–34 (2015).
Sumida, K. et al. Prognostic significance of pre-end-stage renal disease serum alkaline phosphatase for post-end-stage renal disease mortality in late-stage chronic kidney disease patients transitioning to dialysis. Nephrol. Dial. Transplant. http://dx.doi.org/10.1093/ndt/gfw412 (2017).
Bretaudiere, J. P. et al. Criteria for establishing a standardized method for determining alkaline phosphatase activity in human serum. Clin. Chem. 23, 2263–2274 (1977).
Tietz, N. W. et al. Transferability studies for the AACC reference method and the IFCC method for measurement of alkaline phosphatase activity. Clin. Chem. 30, 704–706 (1984).
Kidney Disease: Improving Global Outcomes (KDIGO) CKD–MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int. Suppl. 76, S1–S130 (2009).
Kobayashi, I. et al. Higher serum bone alkaline phosphatase as a predictor of mortality in male hemodialysis patients. Life Sci. 90, 212–218 (2012).
Sardiwal, S. et al. Bone-specific alkaline phosphatase concentrations are less variable than those of parathyroid hormone in stable hemodialysis patients. Kidney Int. 82, 100–105 (2012).
Gardham, C. et al. Variability of parathyroid hormone and other markers of bone mineral metabolism in patients receiving hemodialysis. Clin. J. Am. Soc. Nephrol. 5, 1261–1267 (2010).
Garrett, G., Sardiwal, S., Lamb, E. J. & Goldsmith, D. J. PTH — a particularly tricky hormone: why measure it at all in kidney patients? Clin. J. Am. Soc. Nephrol. 8, 299–312 (2013).
Lamb, E. J. & Delaney, M. P. Does PTH offer additive value to ALP measurement in assessing CKD-MBD? Perit. Dial. Int. 34, 687–691 (2014).
Kozlenkov, A. et al. Residues determining the binding specificity of uncompetitive inhibitors to tissue-nonspecific alkaline phosphatase. J. Bone Miner. Res. 19, 1862–1872 (2004).
Dahl, R. et al. Discovery and validation of a series of aryl sulfonamides as selective inhibitors of tissue-nonspecific alkaline phosphatase (TNAP). J. Med. Chem. 52, 6919–6925 (2009).
Sergienko, E. et al. Identification and characterization of novel tissue-nonspecific alkaline phosphatase inhibitors with diverse modes of action. J. Biomol. Screen. 14, 824–837 (2009).
Sidique, S. et al. Design and synthesis of pyrazole derivatives as potent and selective inhibitors of tissue-nonspecific alkaline phosphatase (TNAP). Bioorg. Med. Chem. Lett. 19, 222–225 (2009).
Debray, J. et al. Inhibitors of tissue-nonspecific alkaline phosphatase: design, synthesis, kinetics, biomineralization and cellular tests. Bioorg. Med. Chem. 21, 7981–7987 (2013).
Marques, S., Buchet, R., Popowycz, F., Lemaire, M. & Mebarek, S. Synthesis of benzofuran derivatives as selective inhibitors of tissue-nonspecific alkaline phosphatase: effects on cell toxicity and osteoblast-induced mineralization. Bioorg. Med. Chem. Lett. 26, 1457–1459 (2016).
Picaud, S. et al. RVX-208, an inhibitor of bet transcriptional regulators with selectivity for the second bromodomain. Proc. Natl Acad. Sci. USA 110, 19754–19759 (2013).
Gilham, D. et al. RVX-208, a bet-inhibitor for treating atherosclerotic cardiovascular disease, raises Apoa-I/HDL and represses pathways that contribute to cardiovascular disease. Atherosclerosis 247, 48–57 (2016).
Kalantar-Zadeh, K. et al. Alkaline phosphatase lowering by selective bet inhibition, a novel mechanism for mace reduction in high risk cvd, diabetes and CKD patients — a post-hoc analysis of phase 2b studies with RVX-208. J. Am. Soc. Nephrol. 26, 227A (2015).
Wong, N. C. W. et al. Apabetalone (RVX-208), a selective bromodomain and extra-terminal (bet) protein inhibitor, decreases abundance and activity of complement proteins in vitro, in mice and in clinical studies. Nephrol. Dial. Transplant. 31, i109 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02586155 (2016)
Whyte, M. P. et al. Enzyme-replacement therapy in life-threatening hypophosphatasia. N. Engl. J. Med. 366, 904–913 (2012).
Whyte, M. P. et al. Asfotase alfa treatment improves survival for perinatal and infantile hypophosphatasia. J. Clin. Endocrinol. Metab. 101, 334–342 (2016).
Heemskerk, S. et al. Alkaline phosphatase treatment improves renal function in severe sepsis or septic shock patients. Crit. Care Med. 37, 417–423 (2009).
Kats, S. et al. Anti-inflammatory effects of alkaline phosphatase in coronary artery bypass surgery with cardiopulmonary bypass. Recent Pat. Inflamm. Allergy Drug Discov. 3, 214–220 (2009).
Peters, E. et al. Pharmacokinetic modeling and dose selection in a randomized, double-blind, placebo-controlled trial of a human recombinant alkaline phosphatase in healthy volunteers. Clin. Pharmacokinet. 55, 1227–1237 (2016).
Ghosh, S. S., Gehr, T. W. & Ghosh, S. Curcumin and chronic kidney disease (CKD): major mode of action through stimulating endogenous intestinal alkaline phosphatase. Molecules 19, 20139–20156 (2014).
Ghosh, S. S., Bie, J., Wang, J. & Ghosh, S. Oral supplementation with non-absorbable antibiotics or curcumin attenuates Western diet-induced atherosclerosis and glucose intolerance in LDLR−/− mice — role of intestinal permeability and macrophage activation. PLoS ONE 9, e108577 (2014).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. M.H. wrote the article. M.H., K.K.-Z. and P.M. provided the figures. M.H. and P.M. made substantial contributions to discussions of the content. All authors contributed to discussion of the content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Isozymes
-
Proteins encoded by separate genes with similar catalytic specificity but different primary structure.
- Isoforms
-
Variations of a protein that arise from single nucleotide polymorphisms, differential splicing of mRNA, or post-translational modifications such as glycosylation.
- Syncytiotrophoblast
-
Epithelial layer that covers the highly vascular embryonic placental villi, which invades the wall of the uterus to establish nutrient circulation between the embryo and the mother.
- Metabolic syndrome
-
Array of conditions — raised blood pressure, dyslipidaemia (raised triglycerides and lowered HDL cholesterol), raised fasting glucose, and increased waist circumference — that increase the risk of cardiovascular disease and type 2 diabetes mellitus.
- Hypophosphatasia
-
Autosomal dominant or autosomal recessive rare metabolic disease with an extraordinary range of severity caused by loss-of-function mutations within ALPL, which encodes TNALP.
Rights and permissions
About this article
Cite this article
Haarhaus, M., Brandenburg, V., Kalantar-Zadeh, K. et al. Alkaline phosphatase: a novel treatment target for cardiovascular disease in CKD. Nat Rev Nephrol 13, 429–442 (2017). https://doi.org/10.1038/nrneph.2017.60
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2017.60
This article is cited by
-
Ratio-fluorescent and naked-eye visualized dual-channel sensing strategy for Cu2+ and alkaline phosphatase activity assay
Analytical Sciences (2024)
-
Response to oral iron therapy in children with anemia of chronic kidney disease
Pediatric Nephrology (2024)
-
Association between changes in serum alkaline phosphatase levels and radiographic progression in ankylosing spondylitis
Scientific Reports (2023)
-
O-Linked N-Acetylglucosamine Transferase Regulates Bone Homeostasis Through Alkaline Phosphatase Pathway in Diabetic Periodontitis
Molecular Biotechnology (2023)
-
Association between serum alkaline phosphatase and cardiovascular events in patients with atrial fibrillation
Heart and Vessels (2023)