Key Points
-
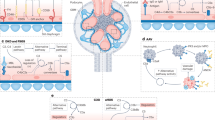
Anti-complement factor H (FH)-associated haemolytic uraemic syndrome (HUS) is characterized by onset at 5–15 years of age, severe illness and marked extrarenal features
-
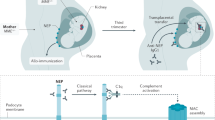
Anti-FH antibodies are mainly directed against the carboxy-terminal part of FH, thereby inhibiting cell surface protection from complement; they are associated with a deficiency in FH-related protein 1 (FHR1) in >80% of cases
-
Plasma exchange is the preferred therapy as it reduces levels of anti-FH antibodies; eculizumab, which blocks the terminal complement pathway, is also effective particularly in refractory cases
-
The combined use of immunosuppression is encouraged as it inhibits the production of antibodies and enables discontinuation of plasma exchange and/or treatment with eculizumab
-
The risk of post-transplantation recurrence is determined by the anti-FH antibody titre and coexisting defects in complement regulation
-
Patients with C3 glomerulopathy might show anti-FH antibodies with amino-terminal specificity, often associated with C3 nephritic factor or monoclonal gammopathy, and without FHR1 deficiency
Abstract
Atypical haemolytic uraemic syndrome (aHUS), an important cause of acute kidney injury, is characterized by dysregulation of the complement pathway, frequent need for dialysis, and progression to end-stage renal disease. Autoantibodies against complement factor H (FH), the main plasma regulatory protein of the alternative pathway of the complement system, account for a considerable proportion of children with aHUS. The autoantibodies are usually associated with the occurrence of a homozygous deletion in the genes encoding the FH-related proteins FHR1 and FHR3. High levels of autoantibodies, noted at the onset of disease and during relapses, induce functional deficiency of FH, whereas their decline, in response to plasma exchanges and/or immunosuppressive therapy, is associated with disease remission. Management with plasma exchange and immunosuppression is remarkably effective in inducing and maintaining remission in aHUS associated with FH autoantibodies, whereas terminal complement blockade with eculizumab is considered the most effective therapy in other forms of aHUS. Anti-FH autoantibodies are also detected in a small proportion of patients with C3 glomerulopathies, which are characterized by chronic glomerular injury mediated by activation of the alternative complement pathway and predominant C3 deposits on renal histology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Walport, M. J. Complement. First of two parts. N. Engl. J. Med. 344, 1058–1066 (2001).
Mele, C., Remuzzi, G. & Noris, M. Hemolytic uremic syndrome. Semin. Immunopathol. 36, 399–420 (2014).
Cerda, J. et al. Epidemiology of acute kidney injury. Clin. J. Am. Soc. Nephrol. 3, 881–886 (2008).
Loirat, C. & Frémeaux-Bacchi, V. Atypical hemolytic uremic syndrome. Orphanet J. Rare Dis. 6, 60 (2011).
Sethi, S. & Fervenza, F. C. Membranoproliferative glomerulonephritis — a new look at an old entity. N. Engl. J. Med. 366, 1119–1131 (2012).
Pickering, M. C. et al. C3 glomerulopathy: consensus report. Kidney Int. 84, 1079–1089 (2013).
Servais, A. et al. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 82, 454–464 (2012).
Zipfel, P. F. et al. The role of complement in C3 glomerulopathy. Mol. Immunol. 67, 21–30 (2015).
Servais, A. et al. Heterogeneous pattern of renal disease associated with homozygous factor H deficiency. Hum. Pathol. 42, 1305–1311 (2011).
Rodríguez de Córdoba, S., Esparza-Gordillo, J., Goicoechea de Jorge, E., Lopez-Trascasa, M. & Sánchez-Corral, P. The human complement factor H: functional roles, genetic variations and disease associations. Mol. Immunol. 41, 355–367 (2004).
Wu, J., Wu, Y. Q., Ricklin, D., Janssen, B. J. & Lambris, J. D. & Gros, P. Structure of complement fragment C3b–factor H and implications for host protection by complement regulators. Nat. Immunol. 10, 728–733 (2009).
Roversi, P. et al. Structural basis for complement factor I control and its disease-associated sequence polymorphisms. Proc. Natl Acad. Sci. USA 108, 12839–12844 (2011).
Bhattacharjee, A., Lehtinen, M. J., Kajander, T., Goldman, A. & Jokiranta, T. S. Both domain 19 and domain 20 of factor H are involved in binding to complement C3b and C3d. Mol. Immunol. 47, 1686–1691 (2010).
Kajander, T. et al. Dual interaction of factor H with C3d and glycosaminoglycans in host-nonhost discrimination by complement. Proc. Natl Acad. Sci. USA 108, 2897–2902 (2011).
Morgan, H. P. et al. Crystallographic determination of the disease-associated T1184R variant of complement regulator factor H. Acta Crystallogr. D Biol. Crystallogr. 67, 593–600 (2011).
Dragon-Durey, M. A. et al. Anti-factor H autoantibodies associated with atypical hemolytic uremic syndrome. J. Am. Soc. Nephrol. 16, 555–563 (2005).
Abarrategui-Garrido, C. et al. Characterization of complement factor H-related (CFHR) proteins in plasma reveals novel genetic variations of CFHR1 associated with atypical hemolytic uremic syndrome. Blood 114, 4261–4271 (2009).
Sinha, A. et al. Prompt plasma exchanges and immunosuppressive treatment improves the outcomes of anti-factor H autoantibody-associated hemolytic uremic syndrome in children. Kidney Int. 85, 1151–1160 (2014).
Hofer, J. et al. Complement factor H-related protein 1 deficiency and factor H antibodies in pediatric patients with atypical hemolytic uremic syndrome. Clin. J. Am. Soc. Nephrol. 8, 407–415 (2013).
Lee, M. et al. Atypical hemolytic uremic syndrome: a Korean pediatric series. Pediatr. Int. 57, 431–438 (2014).
Dragon-Durey, M. A. et al. Clinical features of anti-factor H autoantibody-associated hemolytic uremic syndrome. Clin. J. Am. Soc. Nephrol. 21, 2180–2187 (2010).
Moore, I. et al. Association of factor H autoantibodies with deletions of CFHR1, CFHR3, CFHR4 and with mutations in CFH, CFI, CD46, and C3 in patients with atypical hemolytic uremic syndrome. Blood 115, 379–387 (2010).
Noris, M. et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin. J. Am. Soc. Nephrol. 5, 1844–1859 (2010).
Geerdink, L. M. et al. Atypical hemolytic uremic syndrome in children: complement mutations and clinical characteristics. Pediatr. Nephrol. 27, 1283–1291 (2012).
Jozsi, M. et al. Factor H autoantibodies in atypical hemolytic uremic syndrome correlate with CFHR1/CFHR3 deficiency. Blood 111, 1512–1514 (2008).
Zipfel, P. F. et al. Deletion of complement factor H-related genes CFHR1 and CFHR3 is associated with atypical hemolytic uremic syndrome. PLoS Genet. 3, e41 (2007).
Fremeaux-Bacchi, V. et al. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin. J. Am. Soc. Nephrol. 8, 554–562 (2013).
Dragon-Durey, M. A. et al. The high frequency of complement factor H related CFHR1 gene deletion is restricted to specific subgroups of patients with atypical hemolytic uremic syndrome. J. Med. Genet. 46, 447–450 (2009).
Westra, D. et al. Genetic disorders in complement (regulating) genes in patients with atypical hemolytic uremic syndrome (aHUS). Nephrol. Dial. Transplant. 25, 2195–2202 (2010).
Zipfel, P. F. et al. DEAP-HUS: deficiency of CFHR plasma proteins and autoantibody-positive form of hemolytic uremic syndrome. Pediatr. Nephrol. 25, 2009–2019 (2010).
Strobel, S. et al. Functional analyses indicate a pathogenic role of factor H autoantibodies in atypical hemolytic uremic syndrome. Nephrol. Dial. Transplant. 25, 136–144 (2010).
Guan, N. et al. Clinical characteristics of atypical hemolytic uremic syndrome associated with H factor antibody in children. Zhonghua Er Ke Za Zhi 52, 223–226 (in Chinese) (2014).
Khandelwal, P. et al. Effect of plasma exchange and immunosuppressive medications on antibody titers and outcome in anti-complement factor H antibody-associated hemolytic uremic syndrome. Pediatr. Nephrol. 30, 451–457 (2015).
Kavanagh, D. et al. Factor I autoantibodies in patients with atypical hemolytic uremic syndrome: disease-associated or an epiphenomenon? Clin. J. Am. Soc. Nephrol. 7, 417–426 (2012).
Spitzer, R. E., Stitzel, A. E. & Tsokos, G. C. Production of IgG and IgM autoantibody to the alternative pathway C3 convertase in normal individuals and patients with membranoproliferative glomerulonephritis. Clin. Immunol. Immunopathol. 57, 10–18 (1990).
Goodship, T. H. et al. Factor H autoantibodies in membranoproliferative glomerulonephritis. Mol. Immunol. 52, 200–206 (2012).
Nozal, P. et al. Anti-factor H antibody affecting factor H cofactor activity in a patient with dense deposit disease. Clin. Kidney J. 5, 133–136 (2012).
Blanc, C. et al. Anti-factor H autoantibodies in C3 glomerulopathies and in atypical hemolytic uremic syndrome: one target, two diseases. J. Immunol. 194, 5129–5138 (2015).
Ohi, H. & Yasugi, T. Occurrence of C3 nephritic factor and C4 nephritic factor in membranoproliferative glomerulonephritis (MPGN). Clin. Exp. Immunol. 95, 316–321 (1994).
Strobel, S., Zimmering, M., Papp, K., Prechl, J. & Józsi, M. Anti-factor B autoantibody in dense deposit disease. Mol. Immunol. 47, 1476–1483 (2010).
Chen, Q. et al. Combined C3b and factor B autoantibodies and MPGN type II. N. Eng. J. Med. 365, 2340–2342 (2011).
Bernabéu-Herrero, M. E. et al. Complement factor H, FHR-3 and FHR-1 variants associate in an extended haplotype conferring increased risk of atypical hemolytic uremic syndrome. Mol. Immunol. 67, 276–286 (2015).
Matsukuma, E. et al. A case of atypical hemolytic uremic syndrome due to anti-factor H antibody in a patient presenting with a factor XII deficiency identified two novel mutations. Clin. Exp. Nephrol. 15, 269–274 (2011).
Noone, D. et al. Successful treatment of DEAP-HUS with eculizumab. Pediatr. Nephrol. 29, 841–851 (2014).
Chiodini, B. D. et al. Eculizumab in anti-factor H antibodies associated with atypical hemolytic uremic syndrome. Pediatrics 133, e1764–e1768 (2014).
Uslu-Gökceog˘lu, A., Dog˘an, C. S., Çomak, E., Koyun, M. & Akman, S. Atypical hemolytic uremic syndrome due to factor H autoantibody. Turk. J. Pediatr. 55, 86–89 (2013).
Ito, N. et al. Efficacy and safety of eculizumab in childhood atypical hemolytic uremic syndrome in Japan. Clin. Exp. Nephrol. 20, 265–272 (2016).
Kwon, T., Belot, A. & Ranchin, B. Varicella as a trigger of atypical hemolyticuremic syndrome associated with complement dysfunction: two cases. Nephrol. Dial. Transplant. 24, 2752–2754 (2009).
Boyer, O. et al. Pulse cyclophosphamide therapy and clinical remission in atypical hemolytic uremic syndrome with anti-complement factor H autoantibodies. Am. J. Kidney Dis. 55, 923–927 (2010).
Lee, B. H. et al. Atypical hemolytic uremic syndrome associated with complement factor H autoantibodies and CFHR1/CFHR3 deficiency. Pediatr. Res. 66, 336–340 (2009).
Le Quintrec, M. et al. Anti-factor H autoantibodies in a fifth renal transplant recipient with atypical hemolytic and uremic syndrome. Am. J. Transplant. 9, 1223–1229 (2009).
Song, D. et al. Overactivation of complement alternative pathway in postpartum atypical hemolytic uremic syndrome patients with renal involvement. Am. J. Reprod. Immunol. 74, 345–356 (2015).
Khandelwal, P., Sinha, A., Hari, P. & Bagga, A. Plasma exchanges and immunosuppression for anti-complement factor H associated hemolytic uremic syndrome. Indian Pediatr. 51, 833–835 (2014).
Kim, J. J. et al. The clinical spectrum of hemolytic uremic syndrome secondary to complement factor H autoantibodies. Clin. Nephrol. 83, 49–56 (2015).
Gupta, A. et al. Diarrhea related hemolytic uremic syndrome: unmasking anti-factor H antibodies. Saudi J. Kidney Dis. Transpl. 22, 1017–1018 (2011).
Malina, M. et al. Peripheral gangrene in children with atypical hemolytic uremic syndrome. Pediatrics 131, e331–e335 (2013).
Ardissino, G. et al. Skin involvement in atypical hemolytic uremic syndrome. Am. J. Kidney Dis. 63, 652–655 (2014).
Rigothier, C. et al. Distal angiopathy and atypical hemolytic uremic syndrome: clinical and functional properties of an anti-factor H IgAλ antibody. Am. J. Kidney Dis. 66, 331–336 (2015).
Patschan, D. et al. Idiopathic combined, autoantibody-mediated ADAMTS-13/factor H deficiency in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome in a 17-year-old woman: a case report. J. Med. Case Rep. 5, 598 (2011).
Amornsiripanitch, N. et al. Complement factor H autoantibodies are associated with early stage NSCLC. Clin. Cancer Res. 16, 3226–3231 (2010).
FoltynZadura, A. et al. Factor H autoantibodies and deletion of Complement Factor H-Related protein-1 in rheumatic diseases in comparison to atypical hemolytic uremic syndrome. Arthritis Res. Ther. 14, R1 (2012).
Jodele, S. et al. Abnormalities in the alternative pathway of complement in children with hematopoietic stem cell transplant-associated thrombotic microangiopathy. Blood 122, 2003–2007 (2013).
Dhillon, B. et al. Complement factor H auto-antibodies and age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 51, 5858–5863 (2010).
Rey-Campos, J., Rubinstein, P. & Rodriguez de Cordoba, S. A physical map of the human regulator of complement activation gene cluster linking the complement genes CR1, CR2, DAF, and C4BP. J. Exp. Med. 167, 664–669 (1988).
Estaller, C., Schwaeble, W., Dierich, M. & Weiss, E. H. Human complement fH: two fH proteins are derived from alternatively spliced transcripts. Eur. J. Immunol. 21, 799–802 (1991).
Skerka, C., Chen, Q., Fremeaux-Bacchi, V. & Roumenina, L. T. Complement factor H related proteins (CFHRs). Mol. Immunol. 56, 170–180 (2013).
Heinen, S. et al. De novo gene conversion in the RCA gene cluster (1q32) causes mutations in complement factor H associated with atypical hemolytic uremic syndrome. Hum. Mutat. 27, 292–293 (2006).
Venables, J. P. et al. Atypical hemolytic uremic syndrome associated with a hybrid complement gene. PLoS Med. 3, e431 (2006).
Hellwage, J. et al. Functional properties of complement fH-related proteins FHR-3 and FHR-4: binding to the C3d region of C3b and differential regulation by heparin. FEBS Lett. 462, 345–352 (1999).
Majno, S. A., Jokiranta, T. S., Huehn, M., Seeberger, H. & Hellwage, J. The human FH-related protein 1 (FHR-1) binds to C3b and heparin: implications for a regulatory role on the level of C3 [abstract]. Mol. Immunol. 40, 174 (2003).
McRae, J. L. et al. Human fH-related protein 5 has cofactor activity, inhibits C3 convertase activity, binds heparin and C-reactive protein, and associates with lipoprotein. J. Immunol. 174, 6250–6256 (2005).
Heinen, S. et al. Factor H related protein 1 (CFHR-1) inhibits complement C5 convertase activity and terminal complex formation. Blood 114, 2439–2447 (2009).
Strobel, S. et al. Factor H-related protein 1 neutralizes anti-factor H autoantibodies in autoimmune hemolytic uremic syndrome. Kidney Int. 80, 397–404 (2011).
Blanc, C. et al. Overall neutralization of complement factor H by autoantibodies in the acute phase of the autoimmune form of atypical hemolytic uremic syndrome. J. Immunol. 189, 3528–3537 (2012).
Bhattacharjee, A. et al. The major autoantibody epitope on factor H in atypical hemolytic uremic syndrome is structurally different from its homologous site in factor H related protein 1 supporting a novel model for induction of autoimmunity in this disease. J. Biol. Chem. 290, 9500–9510 (2015).
Nozal, P. et al. Heterogeneity but individual constancy of epitopes isotypes and avidity of factor H autoantibodies inatypical hemolytic uremic syndrome. Mol. Immunol. 70, 47–55 (2015).
Józsi, M. et al. Anti factor H autoantibodies block C-terminal recognition function of factor H in hemolytic uremic syndrome. Blood 110, 1516–1518 (2007).
Rodriguez, E., Rallapalli, P. M., Osborne, A. J. & Perkins, S. J. New functional and structural insights from updated mutational databases for complement factor H, factor I, membrane cofactor protein and C3. Biosci. Rep. 34, e00146 (2014).
Rodríguez de Córdoba, S., Hidalgo, M. S., Pinto, S. & Tortajada, A. Genetics of atypicalhemolyticuremic syndrome (aHUS). Semin. Thromb. Hemost. 40, 422–430 (2014).
Kopp, A., Strobel, S. & Tortajada, A. Atypical hemolytic uremic syndrome-associated variants and autoantibodies impair binding of factor H and factor H-related protein 1 to pentraxin 3. J. Immunol. 189, 1858–1867 (2012).
Deban, L. et al. Binding of the long pentraxin PTX3 to factor H: interacting domains and function in the regulation of complement activation. J. Immunol. 181, 8433–8440 (2008).
Jarva, H., Jokiranta, T. S., Hellwage, J., Zipfel, P. F. & Meri, S. Regulation of complement activation by C-reactive protein: targeting the complement inhibitory activity of factor H by an interaction with short consensus repeat domains 7 and 8–11. J. Immunol. 163, 3957–3962 (1999).
Gharavi, A. G. et al. Genome wide association study identifies susceptibility loci for IgA nephropathy. Nat. Genet. 43, 321–327 (2011).
Zhao, J. et al. Association of genetic variants in complement factor H and factor H-related genes with systemic lupus erythematosus susceptibility. PLoS Genet. 7, e1002079 (2011).
Holmes, L. V. et al. Determining the population frequency of the CFHR3/CFHR1 deletion at 1q32. PLoS ONE 8, e60352 (2013).
Leban, N. et al. Factor H and CFHR1 polymorphisms associated with atypical hemolytic uremic syndrome are differently expressed in Tunisian and Caucasian populations. Int. J. Immunogenet. 39, 110–113 (2012).
Meri, T. et al. Microbes bind complement inhibitor factor H via a common site. PLoS Pathog. 9, e1003308 (2013).
Watson, R. et al. Standardisation of the factor H autoantibody assay. Immunobiology 219, 9–16 (2014).
Sana, G. et al. Long-term remission of atypical HUS with anti-factor H antibodies after cyclophosphamide pulses. Pediatr. Nephrol. 29, 75–83 (2014).
Ardissino, G. et al. Discontinuation of eculizumab maintenance treatment for atypicalhemolyticuremic syndrome: a report of 10 cases. Am. J. Kidney Dis. 64, 633–637 (2014).
Ng, P. C. & Henikoff, S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 31, 3812–3814 (2003).
Adzhubei, I., Jordan, D. M. & Sunyaev, S. R. Predicting functional effect of human missense mutations using PolyPhen-2. Curr. Protoc. Hum. Genet. 7, 20 (2013).
Roumenina, L. T. et al. A prevalent C3 mutation in HUS patients causes a direct C3 convertase gain of-function. Blood 119, 4182–4191 (2012).
Neumann, H. P. et al. Hemolytic uremic syndrome and mutations of the factor H gene: a registry-based study of German speaking countries. J. Med. Genet. 40, 676–681 (2003).
Duvvari, M. R. et al. Analysis of rare variants in the CFH gene in patients with the cuticulardrusen subtype of age-related macular degeneration. Mol. Vis. 21, 285–292 (2015).
Kavanagh, D. et al. Rare genetic variants in the CFI gene are associated with advanced age-related macular degeneration and commonly result in reduced serum factor I levels. Hum. Mol. Genet. 24, 3861–3870 (2015).
Bu, F. et al. High-throughput genetic testing for thrombotic microangiopathies and C3 glomerulopathies. J. Am. Soc. Nephrol. 27, 1245–1253 (2016).
Martinez-Barricarte, R. et al. The complement factor H R1210C mutation is associated with atypical hemolytic uremic syndrome. J. Am. Soc. Nephrol. 19, 639–646 (2008).
KDIGO AKI Work Group. KDIGO clinical practice guidelines for acute kidney injury. Kidney Int. Suppl. 2, 1–138 (2012).
Kwon, T. et al. Successful pre-transplant management of a patient with anti-factor H autoantibodies-associated hemolyticuremic syndrome. Nephrol. Dial. Transplant. 23, 2088–2090 (2008).
Skerka, C. et al. The autoimmune disease DEAP-hemolytic uremic syndrome. Semin. Thromb. Hemost. 36, 625–632 (2010).
Waters, A. M. et al. Successful renal transplantation in factor H autoantibody associated HUS with CFHR1 and 3 deficiency and CFH variant G2850T. Am. J. Transplant. 10, 168–172 (2010).
Lionet, A. et al. A case of adult atypical hemolytic uremic syndrome related to anti-factor H autoantibodies successfully treated by plasma exchange, corticosteroids and rituximab. NDT Plus 2, 458–460 (2009).
Williams, M. E. & Balogun, R. A. Principles of separation: indications and therapeutic targets for plasma exchange. Clin. J. Am. Soc. Nephrol. 9, 181–190 (2014).
Fakhouri, F. et al. Insights from the use in clinical practice of eculizumab in adult patients with atypical hemolytic uremic syndrome affecting the native kidneys: an analysis of 19 cases. Am. J. Kidney Dis. 63, 40–48 (2014).
Johnson, S. et al. An audit analysis of a guideline for the investigation and initial therapy of diarrhea negative (atypical) hemolytic uremic syndrome. Pediatr. Nephrol. 29, 1967–1978 (2014).
Legendre, C. M. et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 368, 2169–2181 (2013).
Licht, C. et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int. 87, 1061–1073 (2015).
Greenbaum, L. A. et al. Eculizumab inhibits thrombotic microangiopathy and improves renal function in pediatric atypical hemolytic uremic syndromepatients [abstract]. J. Am. Soc. Nephrol. 24, 821A–822A (2013).
Fakhouri, F. et al. Eculizumab inhibits thrombotic microangiopathy, and improves renal function in adult atypical hemolytic uremic syndrome patients: 1-year update [abstract]. J. Am. Soc. Nephrol. 25, 751A (2014).
Loirat, C. et al. An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr. Nephrol. 31, 15–39 (2015).
Green, H. et al. Atypical HUS due to factor H antibodies in an adult patient successfully treated with eculizumab. Ren. Fail. 36, 1119–1121 (2014).
Hisano, M. et al. Autoimmune-type atypical hemolytic uremic syndrome treated with eculizumab as first-line therapy. Pediatr. Int. 57, 313–317 (2015).
Zuber, J. et al. Eculizumab for atypical hemolytic uremic syndrome recurrence in renal transplantation. Am. J. Transplant. 12, 3337–3354 (2012).
Buttner-Mainik, A. et al. Production of biologically active recombinant human factor H in Physcomitrella. Plant Biotechnol. J. 9, 373–383 (2011).
Hebecker, M. et al. An engineered construct combining complement regulatory and surface-recognition domains represents a minimal-size functional factor H. J. Immunol. 191, 912–921 (2013).
Schmidt, C. Q. et al. Rational engineering of a minimized immune inhibitor with unique triple-targeting properties. J. Immunol. 190, 5712–5721 (2013).
Lorcy, N., Rioux-Leclercq, N., Lombard, M. L., Le Pogamp, P. & Vigneau, C. Three kidneys, two diseases, one antibody? Nephrol. Dial. Transplant. 26, 3811–3813 (2011).
Khandelwal, P. et al. Outcomes of renal transplant in patients with anti-complement factor H antibody-associated hemolytic uremic syndrome. Pediatr. Transplant. 8, e134–e139 (2014).
Hofer, J. et al. Successful living-related renaltransplantationin a patient with factor H antibody-associated atypical hemolytic uremic syndrome. Pediatr. Transplant. 19, e121–e125 (2015).
Grenda, R. et al. Favorable four-yr outcome after renaltransplantationin a patient with complement factor H antibody and CFHR1/CFHR3 gene mutation-associated HUS. Pediatr. Transplant. 1, e130–e134 (2015).
Zuber, J. et al. Targeted strategies in the prevention and management of atypical HUS recurrence after kidney transplantation. Transplant. Rev. 27, 117–125 (2013).
Józsi, M. et al. Autoantibodies to complement components in C3 glomerulopathy and atypical hemolytic uremic syndrome. Immunol. Lett. 160, 163–171 (2014).
Watson, R. et al. Autoantibodies to CD59, CD55, CD46 or CD35 are not associated with atypical hemolytic uremic syndrome (aHUS). Mol. Immunol. 63, 287–296 (2015).
Meri, S., Koistinen, V., Miettinen, A., Tornroth, T. & Seppala, I. J. T. Activation of the alternative pathway of complement by monoclonal λ light chains in membranoproliferative glomerulonephritis. J. Exp. Med. 175, 939–950 (1992).
Jokiranta, T. S., Solomon, A., Pangburn, M. K., Zipfel, P. F. & Meri, S. Nephritogenic λ light chain dimer: a unique human miniautoantibody against complement factor H. J. Immunol. 163, 4590–4596 (1999).
Sethi, S. et al. Proliferative glomerulonephritis secondary to dysfunction of the alternative pathway of complement. Clin. J. Am. Soc. Nephrol. 6, 1009–1017 (2011).
Bijoux, F. et al. Glomerulonephritis with isolated C3 deposits and monoclonal gammopathy: a fortuitous association? Clin. J. Am. Soc. Nephrol. 6, 2165–2174 (2011).
Payette, A. et al. A case of C3 glomerulonephritis successfully treated with eculizumab. Pediatr. Nephrol. 30, 1033–1037 (2015).
Brackman, D. et al. Thrombotic microangiopathy mimicking membranoproliferative glomerulonephritis. Nephrol. Dial. Transplant. 26, 3399–3403 (2011).
Zhang, Y. et al. Causes of alternative pathway dysregulation in dense deposit disease. Clin. J. Am. Soc. Nephrol. 7, 265–274 (2012).
Sethi, S. et al. Dense deposit disease associated with monoclonal gammopathy of undetermined significance. Am. J. Kidney Dis. 56, 977–982 (2010).
Dragon-Durey, M. A. et al. Heterozygous and homozygous factor H deficiencies associated with hemolytic uremic syndrome or membranoproliferative glomerulonephritis: report and genetic analysis of 16 cases. J. Am. Soc. Nephrol. 15, 787–795 (2004).
Zand, L. et al. C3 glomerulonephritis associated with monoclonal gammopathy: a case series. Am. J. Kidney Dis. 62, 506–514 (2013).
Leung, N. et al. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood 120, 4292–4295 (2012).
Martinez-Barricarte, R. et al. Human C3 mutation reveals a mechanism of dense deposit disease pathogenesis and provides insights into complement activation and regulation. J. Clin. Invest. 120, 3702–3712 (2010).
Abrera-Abeleda, M. A. et al. Variations in the complement regulatory genes factor H (CFH) and factor H related 5 (CFHR5) are associated with membranoproliferative glomerulonephritis type II (dense deposit disease). J. Med. Genet. 43, 582–589 (2006).
Laine, M. et al. Y402H polymorphism of complement factor H affects binding affinity to C-reactive protein. J. Immunol. 178, 3831–3836 (2007).
De Vriese, A. S., Sethi, S., Van Praet, J., Nath, K. A. & Fervenza, F. C. Kidney disease caused by dysregulation of the complement alternative pathway: an etiologic approach. J. Am. Soc. Nephrol. 26, 2917–2929 (2015).
Noris, M. & Remuzzi, G. Glomerular diseases dependent on complement activation, including atypical hemolytic uremic syndrome, membranoproliferative glomerulonephritis, and C3 glomerulopathy: core curriculum 2015. Am. J. Kidney Dis. 66, 359–375 (2015).
Author information
Authors and Affiliations
Contributions
All authors contributed to researching data for the article, discussion of the article's content, writing and reviewing or editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
Frequencies of autoimmune HUS and FHR1 protein deficiency in large series of patients with atypical HUS and healthy controls (PDF 142 kb)
Supplementary information S2 (figure)
Age distribution of patients with anti-FH-associated HUS in various cohorts. (PDF 313 kb)
Supplementary information S3 (figure)
Epitope specificity of anti-FH antibodies in patients with atypical HUS. (PDF 789 kb)
Supplementary information S4 (table)
Concomitant inherited defects in patients with anti-FH-associated HUS, other than deletions of CFHR1 and CFHR3 (PDF 206 kb)
Supplementary information S5 (table)
Outcomes of anti-FH-associated HUS in relation to therapies (PDF 220 kb)
Supplementary information S6 (table)
Clinical features and outcome in patients with anti-FH antibody associated HUS managed with eculizumab (PDF 170 kb)
Supplementary information S7 (table)
Clinical characteristics of patients with C3 glomerulopathy with anti-FH antibodies* (PDF 204 kb)
Rights and permissions
About this article
Cite this article
Durey, MA., Sinha, A., Togarsimalemath, S. et al. Anti-complement-factor H-associated glomerulopathies. Nat Rev Nephrol 12, 563–578 (2016). https://doi.org/10.1038/nrneph.2016.99
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2016.99
This article is cited by
-
Managing anti-factor H antibody-associated hemolytic uremic syndrome: time for consensus
Pediatric Nephrology (2024)
-
Abbreviated protocol of plasma exchanges for patients with anti-factor H associated hemolytic uremic syndrome
Pediatric Nephrology (2024)
-
The role of complement in kidney disease
Nature Reviews Nephrology (2023)
-
Anti-factor H antibody-positive C3 glomerulonephritis secondary to poststreptococcal acute glomerulonephritis with diabetic nephropathy
CEN Case Reports (2023)
-
Variants in complement genes are uncommon in patients with anti-factor H autoantibody-associated atypical hemolytic uremic syndrome
Pediatric Nephrology (2023)