Abstract
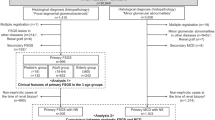
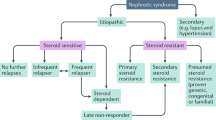
Minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS) are the key histological findings in patients with idiopathic nephrotic syndrome (INS). Although MCD and idiopathic FSGS are often considered to represent separate entities based on differences in their presenting characteristics, histology and outcomes, little evidence exists for this separation. We propose that MCD and idiopathic FSGS are different manifestations of the same progressive disease. The gradual development of FSGS in patients with non-remitting or relapsing INS has been well documented. Moreover, FSGS is the uniform result of substantial podocyte loss in animal models, and a common feature of virtually all progressive human glomerulopathies. As evidence suggests a common aetiology, the pathogenesis of MCD and idiopathic FSGS should be studied together. In clinical trials, idiopathic FSGS should be considered to represent an advanced stage of disease progression that is less likely to respond to treatment than the earlier stage of disease, which is usually defined as MCD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Mason, P. D. & Hoyer, P. F. in Comprehensive Clinical Nephrology (ed. Floege, J.) 218–227 (Elsevier, 2010).
Appel, G. B. & D'Agati, V. in Comprehensive Clinical Nephrology (ed. Floege, J.) 229–240 (Elsevier, 2010).
Fogo, A. B. Causes and pathogenesis of focal segmental glomerulosclerosis. Nat. Rev. Nephrol. 11, 76–87 (2015).
Schwartz, M. M. & Korbet, S. M. Primary focal and segmental glomerulosclerosis: pathology, histological variants, and pathogenesis. Am. J. Kidney Dis. 22, 874–883 (1993).
D'Agati, V. D., Fogo, A. B., Bruijn, J. A. & Jennette, J. C. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am. J. Kidney Dis. 43, 368–382 (2004).
Rennke, H. & Klein, P. S. Pathogenesis and significance of nonprimary focal and segmental glomerulosclerosis. Am. J. Kidney Dis. 13, 443–456 (1989).
Deegens, J. K., Steenbergen, E. J. & Wetzels, J. F. Review on diagnosis and treatment of focal segmental glomerulosclerosis. Neth. J. Med. 66, 3–12 (2008).
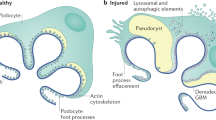
Deegens, J. K. et al. Podocyte foot process effacement as a diagnostic tool in focal segmental glomerulosclerosis. Kidney Int. 74, 1568–1576 (2008).
Churg, J., Habib, R. & White, R. H. Pathology of the nephrotic syndrome in children: a report for the International Study of Kidney Disease in Children. Lancet 760, 1299–1302 (1970).
White, R. H., Glasgow, E. F. & Mills, R. J. Clinicopathological study of nephrotic syndrome in childhood. Lancet 1, 1353–1359 (1970).
Wiggins, R. C. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 71, 1205–1214 (2007).
Kriz, W., Gretz, N. & Lemley, K. V. Progression of glomerular diseases: is the podocyte the culprit? Kidney Int. 54, 687–697 (1998).
Munk, F. Pathologie und klinik der Nephrosen, Nephritiden und Schrumpfnieren (Urban & Schwarzenberg, 1918).
Fahr, T. in Harnorgane Männliche Geschlechtsorgane (ed. Fahr, T.) 156–472 (Springer Vienna, 1925).
Rich, A. R. A hitherto undescribed vulnerability of the juxtamedullary glomeruli in lipoid nephrosis. Bull. Johns Hopkins Hosp. 100, 173–186 (1957).
Hyman, L. R. & Burkholder, P. M. Focal sclerosing glomerulonephropathy with hyalinosis. A clinical and pathologic analysis of the disease in children. J. Pediatr. 84, 217–225 (1974).
Habib, R. Focal glomerular sclerosis. Kidney Int. 4, 355–361 (1973).
Hayslett, J. P., Krassner, L. S., Bensch, K. G., Kashgarian, M. & Epstein, F. H. Progression of “lipoid nephrosis” to renal insufficiency. N. Engl. J. Med. 281, 181–187 (1969).
Tejani, A. Morphological transition in minimal change nephrotic syndrome. Nephron 39, 157–159 (1985).
Maas, R. J., Deegens, J. K., van den Brand, J. A., Cornelissen, E. A. & Wetzels, J. F. A retrospective study of focal segmental glomerulosclerosis: clinical criteria can identify patients at high risk for recurrent disease after first renal transplantation. BMC Nephrol. 14, 47 (2013).
Deegens, J. K., Andresdottir, M. B., Croockewit, S. & Wetzels, J. F. Plasma exchange improves graft survival in patients with recurrent focal glomerulosclerosis after renal transplant. Transpl. Int. 17, 151–157 (2004).
Artero, M., Sharma, R., Savin, V. J. & Vincenti, F. Plasmapheresis reduces proteinuria and serum capacity to injure glomeruli in patients with recurrent focal glomeruloclerosis. Am. J. Kidney Dis. 23, 574–581 (1994).
Canaud, G. et al. Recurrence of nephrotic syndrome after transplantation in a mixed population of children and adults: course of glomerular lesions and value of the Columbia classification of histological variants of focal and segmental glomerulosclerosis (FSGS). Nephrol. Dial. Transplant. 25, 1321–1328 (2010).
Ijpelaar, D. H. et al. Fidelity and evolution of recurrent FSGS in renal allografts. J. Am. Soc. Nephrol. 19, 2219–2224 (2008).
Artz, M. A., Dooper, P. M., Meuleman, E. J., van der Vliet, J. A. & Wetzels, J. F. Time course of proteinuria after living-donor kidney transplantation. Transplantation 76, 421–423 (2003).
Fatima, H. et al. Parietal epithelial cell activation marker in early recurrence of FSGS in the transplant. Clin. J. Am. Soc. Nephrol. 7, 1852–1858 (2012).
Smeets, B. et al. Detection of activated parietal epithelial cells on the glomerular tuft distinguishes early focal segmental glomerulosclerosis from minimal change disease. Am. J. Pathol. 184, 3239–3248 (2014).
Dumoulin, A., Hill, G. S., Montseny, J. J. & Meyrier, A. Clinical and morphological prognostic factors in membranous nephropathy: significance of focal segmental glomerulosclerosis. Am. J. Kidney Dis. 41, 38–48 (2003).
Gupta, R. et al. Focal segmental glomerulosclerosis in idiopathic membranous glomerulonephritis: a clinico-pathological and stereological study. Nephrol. Dial. Transplant. 25, 444–449 (2010).
Rao, T. K. et al. Associated focal and segmental glomerulosclerosis in the acquired immunodeficiency syndrome. N. Engl. J. Med. 310, 669–673 (1984).
Meehan, S. M., Kim, L. & Chang, A. A spectrum of morphologic lesions of focal segmental glomerulosclerosis by Columbia criteria in human immunodeficiency virus infection. Virchows Arch. 460, 429–435 (2012).
Schachter, M. E. et al. Recurrent focal segmental glomerulosclerosis in the renal allograft: single center experience in the era of modern immunosuppression. Clin. Nephrol. 74, 173–181 (2010).
Matsusaka, T. et al. Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J. Am. Soc. Nephrol. 16, 1013–1023 (2005).
Wharram, B. L. et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J. Am. Soc. Nephrol. 16, 2941–2952 (2005).
Frenk, S., Antonowicz, I., Craig, J. M. & Metcoff, J. Experimental nephrotic syndrome induced in rats by aminonucleoside; renal lesions and body electrolyte composition. Proc. Soc. Exp. Biol. Med. 89, 424–427 (1955).
Bertani, T. et al. Adriamycin-induced nephrotic syndrome in rats: sequence of pathologic events. Lab. Invest. 46, 16–23 (1982).
Smeets, B. et al. Podocyte changes upon induction of albuminuria in Thy-1.1 transgenic mice. Nephrol. Dial. Transplant. 18, 2524–2533 (2003).
Diamond, J. R. & Karnovsky, M. J. Focal and segmental glomerulosclerosis following a single intravenous dose of puromycin aminonucleoside. Am. J. Pathol. 122, 481–487 (1986).
Kim, Y. H. et al. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int. 60, 957–968 (2001).
Le Berre, L. et al. The Buffalo/Mna rat, an animal model of FSGS recurrence after renal transplantation. Transplant. Proc. 33, 3338–3340 (2001).
Le Berre, L. et al. Extrarenal effects on the pathogenesis and relapse of idiopathic nephrotic syndrome in Buffalo/Mna rats. J. Clin. Invest. 109, 491–498 (2002).
Robins, R., Baldwin, C., Aoudjit, L., Gupta, I. R. & Takano, T. The spectrum of nephrotic syndrome from minimal change disease to FSGS correlates with Rac1 activation. J. Am. Soc. Nephrol. 26, abstr. SA-OR053 (2015).
Mundel, P. & Reiser, J. Proteinuria: an enzymatic disease of the podocyte? Kidney Int. 77, 571–580 (2010).
Mentzel, S., van Son, J. P., Dijkman, H. B., Wetzels, J. F. & Assmann, K. J. Induction of albuminuria in mice: synergistic effect of two monoclonal antibodies directed to different domains of aminopeptidase A. Kidney Int. 55, 1335–1347 (1999).
Addis, T. The mechanism of proteinuria. Proc. Natl Acad. Sci. USA 35, 194–198 (1949).
Weening, J. J. et al. The pathophysiology of protein-overload proteinuria. Am. J. Pathol. 129, 64–73 (1987).
Gelberg, H., Healy, L., Whiteley, H., Miller, L. A. & Vimr, E. In vivo enzymatic removal of α 2-→6-linked sialic acid from the glomerular filtration barrier results in podocyte charge alteration and glomerular injury. Lab. Invest. 74, 907–920 (1996).
Reiser, J. et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J. Clin. Invest. 113, 1390–1397 (2004).
Trachtman, H., Del Pizzo, R., Valderrama, E. & Gauthier, B. The renal functional and structural consequences of corticosteroid and angiotensin-converting enzyme inhibitor therapy in chronic puromycin aminonucleoside nephropathy. Pediatr. Nephrol. 4, 501–504 (1990).
Wada, T., Pippin, J. W., Marshall, C. B., Griffin, S. V. & Shankland, S. J. Dexamethasone prevents podocyte apoptosis induced by puromycin aminonucleoside: role of p53 and Bcl-2-related family proteins. J. Am. Soc. Nephrol. 16, 2615–2625 (2005).
Clement, L. C. et al. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat. Med. 17, 117–122 (2011).
Pippin, J. W. et al. Inducible rodent models of acquired podocyte diseases. Am. J. Physiol. Renal Physiol. 296, F213–F229 (2009).
Chugh, S. et al. Aminopeptidase A: a nephritogenic target antigen of nephrotoxic serum. Kidney Int. 59, 601–613 (2001).
Chugh, S. S., Clement, L. C. & Mace, C. New insights into human minimal change disease: lessons from animal models. Am. J. Kidney Dis. 59, 284–292 (2012).
Maas, R. J., Deegens, J. K. & Wetzels, J. F. Permeability factors in idiopathic nephrotic syndrome: historical perspectives and lessons for the future. Nephrol. Dial. Transplant. 29, 2207–2216 (2014).
Wei, C. et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat. Med. 17, 952–960 (2011).
McCarthy, E. T., Sharma, M. & Savin, V. J. Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 5, 2115–2121 (2010).
Koyama, A., Fujisaki, M., Kobayashi, M., Igarashi, M. & Narita, M. A glomerular permeability factor produced by human T cell hybridomas. Kidney Int. 40, 453–460 (1991).
Brenchley, P. E. Vascular permeability factors in steroid-sensitive nephrotic syndrome and focal segmental glomerulosclerosis. Nephrol. Dial. Transplant. 18 (Suppl. 6), vi21–vi25 (2003).
Mansour, H. et al. T-cell transcriptome analysis points up a thymic disorder in idiopathic nephrotic syndrome. Kidney Int. 67, 2168–2177 (2005).
Bakker, W. W. et al. Altered activity of plasma hemopexin in patients with minimal change disease in relapse. Pediatr. Nephrol. 20, 1410–1415 (2005).
Maas, R. J., Wetzels, J. F. & Deegens, J. K. Serum-soluble urokinase receptor concentration in primary FSGS. Kidney Int. 81, 1043–1044 (2012).
Meijers, B. et al. The soluble urokinase receptor is not a clinical marker for focal segmental glomerulosclerosis. Kidney Int. 85, 636–640 (2014).
Wada, T. et al. A multicenter cross-sectional study of circulating soluble urokinase receptor in Japanese patients with glomerular disease. Kidney Int. 85, 641–648 (2014).
Sinha, A. et al. Serum-soluble urokinase receptor levels do not distinguish focal segmental glomerulosclerosis from other causes of nephrotic syndrome in children. Kidney Int. 85, 649–658 (2014).
Spinale, J. M. et al. A reassessment of soluble urokinase-type plasminogen activator receptor in glomerular disease. Kidney Int. 87, 564–574 (2015).
Shalhoub, R. J. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet 2, 556–560 (1974).
Araya, C. et al. T regulatory cell function in idiopathic minimal lesion nephrotic syndrome. Pediatr. Nephrol. 24, 1691–1698 (2009).
Prasad, N. et al. Differential alteration in peripheral T-regulatory and T-effector cells with change in P-glycoprotein expression in Childhood Nephrotic Syndrome: a longitudinal study. Cytokine 72, 190–196 (2015).
Le Berre, L. et al. Induction of T regulatory cells attenuates idiopathic nephrotic syndrome. J. Am. Soc. Nephrol. 20, 57–67 (2009).
Audard, V., Pawlak, A., Candelier, M., Lang, P. & Sahali, D. Upregulation of nuclear factor-related kappa B suggests a disorder of transcriptional regulation in minimal change nephrotic syndrome. PLoS ONE 7, e30523 (2012).
Aviles, D. H., Matti Vehaskari, V., Manning, J., Ochoa, A. C. & Zea, A. H. Decreased expression of T-cell NF-κB p65 subunit in steroid-resistant nephrotic syndrome. Kidney Int. 66, 60–67 (2004).
Lama, G. et al. T-Lymphocyte populations and cytokines in childhood nephrotic syndrome. Am. J. Kidney Dis. 39, 958–965 (2002).
Sellier-Leclerc, A. L. et al. A humanized mouse model of idiopathic nephrotic syndrome suggests a pathogenic role for immature cells. J. Am. Soc. Nephrol. 18, 2732–2739 (2007).
Hinkes, B. G. et al. Nephrotic syndrome in the first year of life: two thirds of cases are caused by mutations in 4 genes (NPHS1, NPHS2, WT1, and LAMB2). Pediatrics 119, e907–e919 (2007).
Autio-Harmainen, H. & Rapola, J. Renal pathology of fetuses with congenital nephrotic syndrome of the Finnish type. a qualitative and quantitative light microscopic study. Nephron 29, 158–163 (1981).
Kaplan, J. M. et al. Mutations in ACTN4, encoding α-actinin-4, cause familial focal segmental glomerulosclerosis. Nat. Genet. 24, 251–256 (2000).
Winn, M. P. et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308, 1801–1804 (2005).
Brown, E. J. et al. Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat. Genet. 42, 72–76 (2010).
Gbadegesin, R. A. et al. Mutations in the gene that encodes the F-actin binding protein anillin cause FSGS. J. Am. Soc. Nephrol. 25, 1991–2002 (2014).
Brown, E. J., Pollak, M. R. & Barua, M. Genetic testing for nephrotic syndrome and FSGS in the era of next-generation sequencing. Kidney Int. 85, 1030–1038 (2014).
Santin, S. et al. Clinical utility of genetic testing in children and adults with steroid-resistant nephrotic syndrome. Clin. J. Am. Soc. Nephrol. 6, 1139–1148 (2011).
Rood, I. M., Deegens, J. K. & Wetzels, J. F. Genetic causes of focal segmental glomerulosclerosis: implications for clinical practice. Nephrol. Dial. Transplant. 27, 882–890 (2012).
Sadowski, C. E. et al. A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J. Am. Soc. Nephrol. 26, 1279–1289 (2015).
Karp, A. M. & Gbadegesin, R. A. Genetics of childhood steroid-sensitive nephrotic syndrome. Pediatr. Nephrol. http://dx.doi.org/10.1007/s00467-016-3456-8 (2016).
Gee, H. Y. et al. Mutations in EMP2 cause childhood-onset nephrotic syndrome. Am. J. Hum. Genet. 94, 884–890 (2014).
Wan, X. et al. Loss of epithelial membrane protein 2 aggravates podocyte injury via upregulation of caveolin-1. J. Am. Soc. Nephrol. 27, 1066–1075 (2016).
Gbadegesin, R. A. et al. HLA-DQA1 and PLCG2 are candidate risk loci for childhood-onset steroid-sensitive nephrotic syndrome. J. Am. Soc. Nephrol. 26, 1701–1710 (2015).
McAdam, A. J., Schweitzer, A. N. & Sharpe, A. H. The role of B7 co-stimulation in activation and differentiation of CD4+ and CD8+ T cells. Immunol. Rev. 165, 231–247 (1998).
Garin, E. H. et al. Urinary CD80 is elevated in minimal change disease but not in focal segmental glomerulosclerosis. Kidney Int. 78, 296–302 (2010).
Cara-Fuentes, G. et al. CD80 and suPAR in patients with minimal change disease and focal segmental glomerulosclerosis: diagnostic and pathogenic significance. Pediatr. Nephrol. 29, 1363–1371 (2014).
Ling, C. et al. Urinary CD80 levels as a diagnostic biomarker of minimal change disease. Pediatr. Nephrol. 30, 309–316 (2014).
Yu, C. C. et al. Abatacept in B7-1-positive proteinuric kidney disease. N. Engl. J. Med. 369, 2416–2423 (2013).
Novelli, R., Gagliardini, E., Ruggiero, B., Benigni, A. & Remuzzi, G. Any value of podocyte B7-1 as a biomarker in human MCD and FSGS? Am. J. Physiol. Renal Physiol. 310, F335–F341 (2016).
Delville, M. et al. B7-1 blockade does not improve post-transplant nephrotic syndrome caused by recurrent FSGS. J. Am. Soc. Nephrol. 27, 2520–2527 (2016).
Larsen, C. P., Messias, N. C. & Walker, P. D. B7-1 immunostaining in proteinuric kidney disease. Am. J. Kidney Dis. 64, 1001–1003 (2014).
Garin, E. H. et al. Case series: CTLA4-IgG1 therapy in minimal change disease and focal segmental glomerulosclerosis. Pediatr. Nephrol. 30, 469–477 (2015).
Regele, H. M. et al. Glomerular expression of dystroglycans is reduced in minimal change nephrosis but not in focal segmental glomerulosclerosis. J. Am. Soc. Nephrol. 11, 403–412 (2000).
Giannico, G., Yang, H., Neilson, E. G. & Fogo, A. B. Dystroglycan in the diagnosis of FSGS. Clin. J. Am. Soc. Nephrol. 4, 1747–1753 (2009).
Vogtlander, N. P. et al. Expression of sialidase and dystroglycan in human glomerular diseases. Nephrol. Dial. Transplant. 25, 478–484 (2010).
Schmid, H. et al. Gene expression profiles of podocyte-associated molecules as diagnostic markers in acquired proteinuric diseases. J. Am. Soc. Nephrol. 14, 2958–2966 (2003).
Hodgin, J. B. et al. A molecular profile of focal segmental glomerulosclerosis from formalin-fixed, paraffin-embedded tissue. Am. J. Pathol. 177, 1674–1686 (2010).
Shankland, S. J., Smeets, B., Pippin, J. W. & Moeller, M. J. The emergence of the glomerular parietal epithelial cell. Nat. Rev. Nephrol. 10, 158–173 (2014).
Bennett, M. R. et al. Laser capture microdissection-microarray analysis of focal segmental glomerulosclerosis glomeruli. Nephron Exp. Nephrol. 107, e30–e40 (2007).
Endlich, N. et al. Analysis of differential gene expression in stretched podocytes: osteopontin enhances adaptation of podocytes to mechanical stress. FASEB J. 16, 1850–1852 (2002).
Hanley, K. P. et al. Ectopic SOX9 mediates extracellular matrix deposition characteristic of organ fibrosis. J. Biol. Chem. 283, 14063–14071 (2008).
International Study of Kidney Disease in Children. Nephrotic syndrome in children: prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. Kidney Int. 13, 159–165 (1978).
Malone, A. F. et al. Rare hereditary COL4A3/COL4A4 variants may be mistaken for familial focal segmental glomerulosclerosis. Kidney Int. 86, 1253–1259 (2014).
Jao, W., Pollak, V. E., Norris, S. H., Lewy, P. & Pirani, C. L. Lipoid nephrosis: an approach to the clinicopathologic analysis and dismemberment of idiopathic nephrotic syndrome with minimal glomerular changes. Medicine (Baltimore) 52, 445–468 (1973).
Fogo, A. et al. Glomerular hypertrophy in minimal change disease predicts subsequent progression to focal glomerular sclerosis. Kidney Int. 38, 115–123 (1990).
Nyberg, E., Bohman, S. O. & Berg, U. Glomerular volume and renal function in children with different types of the nephrotic syndrome. Pediatr. Nephrol. 8, 285–289 (1994).
Suzuki, J., Yoshikawa, N. & Nakamura, H. A quantitative analysis of the glomeruli in focal segmental glomerulosclerosis. Pediatr. Nephrol. 8, 416–419 (1994).
Tsuboi, N. et al. Glomerular density in renal biopsy specimens predicts the long-term prognosis of IgA nephropathy. Clin. J. Am. Soc. Nephrol. 5, 39–44 (2010).
Puelles, V. G. et al. Podocyte number in children and adults: associations with glomerular size and numbers of other glomerular resident cells. J. Am. Soc. Nephrol. 26, 2277–2288 (2015).
Cameron, J. S. & Blandford, G. The simple assessment of selectivity in heavy proteinuria. Lancet 2, 242–247 (1966).
Hanamura, K., Tojo, A. & Fujita, T. Urinary and glomerular podocytes in patients with chronic kidney diseases. Clin. Exp. Nephrol. 18, 95–103 (2014).
Wickman, L. et al. Urine podocyte mRNAs, proteinuria, and progression in human glomerular diseases. J. Am. Soc. Nephrol. 24, 2081–2095 (2013).
Mak, S. K., Short, C. D. & Mallick, N. P. Long-term outcome of adult-onset minimal-change nephropathy. Nephrol. Dial. Transplant. 11, 2192–2201 (1996).
Nolasco, F. et al. Adult-onset minimal change nephrotic syndrome: a long-term follow-up. Kidney Int. 29, 1215–1223 (1986).
Waldman, M. et al. Adult minimal-change disease: clinical characteristics, treatment, and outcomes. Clin. J. Am. Soc. Nephrol. 2, 445–453 (2007).
Cameron, J. S. The long term prognosis of patients with focal glomerulosclerosis. Clin. Nephrol. 10, 213–229 (1978).
Beaufils, H., Alphonse, J. C., Guedon, J. & Legrain, M. Focal glomerulosclerosis: natural history and treatment. A report of 70 cases. Nephron 21, 75–85 (1978).
Banfi, G. et al. The impact of prolonged immunosuppression on the outcome of idiopathic focal-segmental glomerulosclerosis with nephrotic syndrome in adults. A collaborative retrospective study. Clin. Nephrol. 36, 53–59 (1991).
Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int. 2 (Suppl.), 139–274 (2012).
Ding, W. Y. et al. Initial steroid sensitivity in children with steroid-resistant nephrotic syndrome predicts post-transplant recurrence. J. Am. Soc. Nephrol. 25, 1342–1348 (2014).
Coggins, C. H. Adult minimal change nephropathy: experience of the collaborative study of glomerular disease. Trans. Am. Clin. Climatol. Assoc. 97, 18–26 (1986).
Matsusaka, T. et al. Podocyte injury damages other podocytes. J. Am. Soc. Nephrol. 22, 1275–1285 (2011).
Venkatareddy, M. et al. Estimating podocyte number and density using a single histologic section. J. Am. Soc. Nephrol. 25, 1118–1129 (2014).
Acknowledgements
The authors' work is supported by the Dutch Kidney Foundation (OW08, R.J.M and J.F.W.; 14A3D104, B.S.), the Netherlands Organization for Scientific Research (NWO grant 92003587, J.K.D.), the consortium STOP-FSGS by the German Ministry for Science and Education (BMBF 01GM1518A, M.J.M.), and TP17 of the SFB/Transregio 57 “Mechanisms of organ fibrosis” of the German Research Foundation (M.J.M). M.J.M. has also been awarded a Heisenberg Professorship (DFG MO 1082/7-1).
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article, discussed the content and wrote the manuscript. R.J.M. and J.F.W. revised and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Maas, R., Deegens, J., Smeets, B. et al. Minimal change disease and idiopathic FSGS: manifestations of the same disease. Nat Rev Nephrol 12, 768–776 (2016). https://doi.org/10.1038/nrneph.2016.147
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2016.147
This article is cited by
-
Rationale and design of the Japanese Biomarkers in Nephrotic Syndrome (J-MARINE) study
Clinical and Experimental Nephrology (2024)
-
Efficacy of low-dose rituximab in minimal change disease and prevention of relapse
BMC Nephrology (2023)
-
Repetitive administration of rituximab can achieve and maintain clinical remission in patients with MCD or FSGS
Scientific Reports (2023)
-
Kidney biopsy diagnosis in childhood in the Norwegian Kidney Biopsy Registry and the long-term risk of kidney replacement therapy: a 25-year follow-up
Pediatric Nephrology (2023)
-
Diagnostik und Therapie der Minimal Change Glomerulopathie beim Erwachsenen – 2023
Wiener klinische Wochenschrift (2023)