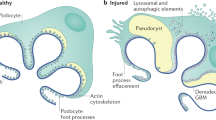
Key Points
-
Primary disease recurrence after renal transplantation accounts for 7–8% of graft losses
-
Disease recurrence after paediatric renal transplantation can be associated with a high or low risk of graft loss depending on the underlying primary disease
-
Understanding of the pathophysiology of most kidney diseases has advanced dramatically in the past few years, which will hopefully lead to improvements in the global prognosis of paediatric renal transplantation
-
Targeted treatment strategies are available for specific diseases that can improve graft survival in patients with disease recurrence
Abstract
Primary disease recurrence after renal transplantation is mainly diagnosed by examination of biopsy samples, but can also be associated with clinical symptoms. In some patients, recurrence can lead to graft loss (7–8% of all graft losses). Primary disease recurrence is generally associated with a high risk of graft loss in patients with focal segmental glomerulosclerosis, membranous proliferative glomerulonephritis, primary hyperoxaluria or atypical haemolytic uraemic syndrome. By contrast, disease recurrence is associated with a limited risk of graft loss in patients with IgA nephropathy, renal involvement associated with Henoch–Schönlein purpura, antineutrophil cytoplasmic antibody-associated glomerulonephritis or lupus nephritis. The presence of systemic diseases that affect the kidneys, such as sickle cell anaemia and diabetes mellitus, also increases the risk of delayed graft loss. This Review provides an overview of the epidemiology, pathophysiology and management of primary disease recurrence in paediatric renal graft recipients, and describes the overall effect on graft survival of each of the primary diseases listed above. With appropriate management, few paediatric patients should be excluded from renal transplantation programmes because of an increased risk of recurrence.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
North American Pediatric Renal Trials and Collaborative Studies. NAPRTCS 2010 Annual Transplant Report. The EMMES Corporation™ [online], (2010).
Cochat, P. et al. Disease recurrence in paediatric renal transplantation. Pediatr. Nephrol. 24, 2097–2108 (2009).
Van Stralen, K. J. et al. Impact of graft loss among kidney diseases with a high risk of post-transplant recurrence in the paediatric population. Nephrol. Dial. Transplant. 28, 1031–1038 (2013).
Holmberg, C. & Jalanko, H. Congenital nephrotic syndrome and recurrence of proteinuria after renal transplantation. Pediatr. Nephrol. 29, 2309–2317 (2014).
Canaud, G. et al. The kidney as a reservoir for HIV1 after renal transplantation. J. Am. Soc. Nephrol. 25, 407–419 (2014).
Mekahli, D. et al. Long-term outcome of idiopathic steroid-resistant nephrotic syndrome: a multicenter study. Pediatr. Nephrol. 24, 1525–1532 (2009).
Harambat, J. et al. Paediatric ESRD: 2013 data from the REIN registry. Société de néphrologie [online], (2013).
Fine, R. N. Recurrence of nephrotic syndrome/focal segmental glomerulosclerosis following renal transplantation in children. Pediatr. Nephrol. 22, 496–502 (2007).
Baum, M. A., Ho, M., Stablein, D. & Alexander, S. R. Outcome of renal transplantation in adolescents with focal segmental glomerulosclerosis. Pediatr. Transplant. 6, 488–492 (2002).
Saleem, M. A. The phenomenon of focal segmental glomerulosclerosis posttransplantationa one-hit wonder? Pediatr. Nephrol. 27, 2163–2166 (2012).
Shankland, S. J. & Pollak, M. R. A suPAR circulating factor causes kidney disease. Nat. Med. 17, 926–927 (2011).
Wei, C. et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat. Med. 17, 952–960 (2011).
Gallon, L., Leventhal, J., Skaro, A., Kanwar, Y. & Alvarado, A. Resolution of recurrent focal segmental glomerulosclerosis after retransplantation. N. Engl. J. Med. 366, 1648–1649 (2012).
Mohey, H., Thibaudin, L., Laurent, B. & Berthoux, F. The podocin mutation R229Q and early recurrence (within the first year) of glomerular disease after renal transplantation. Ann. Transplant. 18, 436–442 (2013).
Bouchireb, K. et al. NPHS2 mutations in steroid-resistant nephrotic syndrome: a mutation update and the associated phenotypic spectrum. Hum. Mutat. 35, 178–186 (2014).
Buscher, A. K. et al. Mutations in podocyte genes are a rare cause of primary FSGS associated with ESRD in adult patients. Clin. Nephrol. 78, 47–53 (2012).
Fujisawa, M. et al. Long term outcome of FSGS after Japanese pediatric renal transplantation. Pediatr. Nephrol. 17, 165–168 (2002).
Couloures, K., Pepkowitz, S. H., Goldfinger, D., Kamil, E. S. & Puliyanda, D. P. Preventing recurrence of focal segmental glomerulosclerosis following renal transplantation: a case report. Pediatr. Transplant. 10, 962–965 (2006).
Gonzalez, E., Ettenger, R., Rianthavorn, P., Tsai, E. & Malekzadeh, M. Preemptive plasmapheresis and recurrence of focal segmental glomerulosclerosis in pediatric renal transplantation. Pediatr. Transplant. 15, 495–501 (2011).
Chikamoto, H. et al. Pretransplantation combined therapy with plasmapheresis and rituximab in a second living-related kidney transplant pediatric recipient with a very high risk for focal segmental glomerulosclerosis recurrence. Pediatr. Transplant. 16, E286–E290 (2012).
Cochat, P. Is there a need for a multicenter study to determine the optimal approach to recurrent nephrotic syndrome following renal transplantation? Pediatr. Transplant. 5, 394–397 (2001).
Fargue, S. et al. Prevention and treatment of recurrence of steroid-resistant nephrotic syndrome after kidney transplantation in children. Pediatr. Nephrol. 23, 1685 (2008).
Kasiske, B. L. et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. 77, 299–311 (2010).
Hodson, E. M., Willis, N. S. & Craig, J. C. Interventions for idiopathic steroid-resistant nephrotic syndrome in children. Cochrane Database of Systematic Reviews, Issue 11. Art. No.: CD003594 (2010).
Vinai, M., Waber, P. & Seikaly, M. G. Recurrence of focal segmental glomerulosclerosis in renal allograft: an in-depth review. Pediatr. Transplant. 14, 314–325 (2010).
Hickson, L. J. et al. Kidney transplantation for primary focal segmental glomerulosclerosis: outcomes and response to therapy for recurrence. Transplantation 87, 1232–1239 (2009).
Grenda, R., Jarmuzek, W., Piatosa, B. & Rubik, J. Long-term effect of rituximab in maintaining remission of recurrent and plasmapheresis-dependent nephrotic syndrome post-renal transplantation—case report. Pediatr. Transplant. 15, E121–E125 (2011).
Meyer, T. N., Thaiss, F. & Stahl, R. A. Immunoadsorbtion and rituximab therapy in a second living-related kidney transplant patient with recurrent focal segmental glomerulosclerosis. Transpl. Int. 20, 1066–1071 (2007).
Kumar, J. et al. Rituximab in post-transplant pediatric recurrent focal segmental glomerulosclerosis. Pediatr. Nephrol. 28, 333–338 (2013).
Bayrakci, U. S., Baskin, E., Sakalli, H., Karakayali, H. & Haberal, M. Rituximab for post-transplant recurrences of FSGS. Pediatr. Transplant. 13, 240–243 (2009).
Stewart, Z. A., Shetty, R., Nair, R., Reed, A. I. & Brophy, P. D. Case report: successful treatment of recurrent focal segmental glomerulosclerosis with a novel rituximab regimen. Transplant. Proc. 43, 3994–3996 (2011).
Cochat, P. et al. Recurrent nephrotic syndrome after transplantation: early treatment with plasmaphaeresis and cyclophosphamide. Pediatr. Nephrol. 7, 50–54 (1993).
Nathanson, S. et al. Recurrence of nephrotic syndrome after renal transplantation: influence of increased immunosuppression. Pediatr. Nephrol. 20, 1801–1804 (2005).
Straatmann, C. et al. Success with plasmapheresis treatment for recurrent focal segmental glomerulosclerosis in pediatric renal transplant recipients. Pediatr. Transplant. 18, 29–34 (2014).
Dantal, J. et al. Effect of plasma protein adsorption on protein excretion in kidney-transplant recipients with recurrent nephrotic syndrome. N. Engl. J. Med. 330, 7–14 (1994).
Fencl, F. et al. Recurrence of nephrotic proteinuria in children with focal segmental glomerulosclerosis after renal transplantation treated with plasmapheresis and immunoadsorption: case reports. Transplant. Proc. 39, 3488–3490 (2007).
Beaudreuil, S. et al. Protein A immunoadsorption cannot significantly remove the soluble receptor of urokinase from sera of patients with recurrent focal segmental glomerulosclerosis. Nephrol. Dial. Transplant. 29, 458–463 (2014).
Kwon, T., Nattes, E. & Deschenes, G. Efficacy of immunoadsorption in pediatric nephrotic syndrome. Presented at the 2014 European Society for Pediatric Nephrology meeting, Porto, Portugal.
Yu, C. C. et al. Abatacept in B7-1-positive proteinuric kidney disease. N. Engl. J. Med. 369, 2416–2423 (2013).
Basu, B. Ofatumumab for rituximab-resistant nephrotic syndrome. N. Engl. J. Med. 370, 1268–1270 (2014).
Sgambat, K., Banks, M. & Moudgil, A. Effect of galactose on glomerular permeability and proteinuria in steroid-resistant nephrotic syndrome. Pediatr. Nephrol. 28, 2131–2135 (2013).
Raafat, R., Travis, L., Kalia, A. & Diven, S. Role of transplant induction. Pediatr. Nephrol. 14, 189–194 (2000).
Little, M. A., Dupont, P., Campbell, E., Dorman, A. & Walshe, J. J. Severity of primary MPGN, rather than MPGN type, determines renal survival and post-transplantation recurrence risk. Kidney Int. 69, 504–511 (2006).
Pickering, M. C. et al. C3 glomerulopathy: consensus report. Kidney Int. 84, 1079–1089 (2013).
Sethi, S., Nester, C. M. & Smith, R. J. H. Membranoproliferative glomerulonephritis and C3 glomerulopathy: resolving the confusion. Kidney Int. 81, 434–441 (2012).
Zand, L. et al. Clinical findings, pathology, and outcomes of C3GN after kidney transplantation. J. Am. Soc. Nephrol. 25, 1110–1117 (2014).
Lorenz, E. C. et al. Recurrent membranoproliferative glomerulonephritis after kidney transplantation. Kidney Int. 77, 721–728 (2010).
Marinaki, S., Lionaki, S. & Boletis, J. N. Glomerular disease recurrence in the renal allograft: a hurdle but not a barrier for successful kidney transplantation. Transplant. Proc. 45, 3–9 (2013).
McCaughan, J. A., O'Rourke, D. M. & Courtney, A. E. Recurrent dense deposit disease after renal transplantation: an emerging role for complementary therapies. Am. J. Transplant. 12, 1046–1051 (2012).
Radhakrishnan, S. et al. Eculizumab and refractory membranoproliferative glomerulonephritis. N. Engl. J. Med. 366, 1165–1166 (2012).
Gurkan, S. et al. Eculizumab and recurrent C3 glomerulonephritis. Pediatr. Nephrol. 28, 1975–1981 (2013).
Nester, C. et al. Pre-emptive eculizumab and plasmapheresis for renal transplant in atypical hemolytic uremic syndrome. Clin. J. Am. Soc. Nephrol. 6, 1488–1494 (2011).
Moroni, G. et al. Long-term outcome of renal transplantation in patients with idiopathic membranous glomerulonephritis (MN). Nephrol. Dial. Transplant. 25, 3408–3415 (2010).
Beck, L. H. Jr et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N. Engl. J. Med. 361, 11–21 (2009).
Debiec, H. et al. Autoantibodies specific for the phospholipase A2 receptor in recurrent and de novo membranous nephropathy. Am. J. Transplant. 11, 2144–2152 (2011).
El-Zoghby, Z. M. et al. Recurrent idiopathic membranous nephropathy: early diagnosis by protocol biopsies and treatment with anti-CD20 monoclonal antibodies. Am. J. Transplant. 9, 2800–2807 (2009).
Rodriguez, E. F. et al. The pathology and clinical features of early recurrent membranous glomerulonephritis. Am. J. Transplant. 12, 1029–1038 (2012).
Andrews, P. A. Lipoprotein glomerulopathy: a new cause of nephrotic syndrome after renal transplantation. Implications for renal transplantation. Nephrol. Dial. Transplant. 14, 239–240 (1999).
Loirat, C. & Fremeaux-Bacchi, V. Hemolytic uremic syndrome recurrence after renal transplantation. Pediatr. Transplant. 12, 619–629 (2008).
Sellier-Leclerc, A. L. et al. Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J. Am. Soc. Nephrol. 18, 2392–2400 (2007).
Kwon, T. et al. Successful pre-transplant management of a patient with anti-factor H autoantibodies-associated haemolytic uraemic syndrome. Nephrol. Dial. Transplant. 23, 2088–2090 (2008).
Loirat, C., Girma, J. P., Desconclois, C., Coppo, P. & Veyradier, A. Thrombotic thrombocytopenic purpura related to severe ADAMTS13 deficiency in children. Pediatr. Nephrol. 24, 19–29 (2009).
Zimmerhackl, L. B. et al. Epidemiology, clinical presentation, and pathophysiology of atypical and recurrent hemolytic uremic syndrome. Semin. Thromb. Hemost. 32, 113–120 (2006).
Zuber, J. et al. Targeted strategies in the prevention and management of atypical HUS recurrence after kidney transplantation. Transplant. Rev. (Orlando) 27, 117–125 (2013).
Le Quintrec, M. et al. Complement genes strongly predict recurrence and graft outcome in adult renal transplant recipients with atypical hemolytic and uremic syndrome. Am. J. Transplant. 13, 663–675 (2013).
Lemaire, M. et al. Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat. Genet. 45, 531–536 (2013).
Bresin, E. et al. Combined complement gene mutations in atypical hemolytic uremic syndrome influence clinical phenotype. J. Am. Soc. Nephrol. 24, 475–486 (2013).
Pabst, W. L. et al. Successful long-term outcome after renal transplantation in a patient with atypical haemolytic uremic syndrome with combined membrane cofactor protein CD46 and complement factor I mutations. Pediatr. Nephrol. 28, 1141–1144 (2013).
Michaux, K. et al. Eculizumab in neonatal hemolytic uremic syndrome with homozygous factor H deficiency. Pediatr. Nephrol. 29, 2415–2419 (2014).
Hirt-Minkowski, P. et al. Haemolytic uraemic syndrome caused by factor H mutation: is single kidney transplantation under intensive plasmatherapy an option? Nephrol. Dial. Transplant. 24, 3548–3551 (2009).
Zuber, J. et al. Eculizumab for atypical hemolytic uremic syndrome recurrence in renal transplantation. Am. J. Transplant. 12, 3337–3354 (2012).
Weitz, M., Amon, O., Bassler, D., Koenigsrainer, A. & Nadalin, S. Prophylactic eculizumab prior to kidney transplantation for atypical hemolytic uremic syndrome. Pediatr. Nephrol. 26, 1325–1329 (2011).
Al-Akash, S. I., Almond, P. S., Savell, V. H. Jr, Gharaybeh, S. I. & Hogue, C. Eculizumab induces long-term remission in recurrent post-transplant HUS associated with C3 gene mutation. Pediatr. Nephrol. 26, 613–619 (2011).
Chatelet, V. et al. Eculizumab: safety and efficacy after 17 months of treatment in a renal transplant patient with recurrent atypical hemolytic-uremic syndrome: case report. Transplant. Proc. 42, 4353–4355 (2010).
Legendre, C. M. et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 368, 2169–2181 (2013).
Roman-Ortiz, E. et al. Eculizumab long-term therapy for pediatric renal transplant in aHUS with CFH/CFHR1 hybrid gene. Pediatr. Nephrol. 29, 149–153 (2014).
Loirat, C., Coppo, P. & Veyradier, A. Thrombotic thrombocytopenic purpura in children. Curr. Opin. Pediatr. 25, 216–224 (2013).
Khandelwal, P. et al. Effect of plasma exchange and immunosuppressive medications on antibody titers and outcome in anti-complement factor H antibody-associated hemolytic uremic syndrome. Pediatr. Nephrol. 30, 451–457 (2015).
Khandelwal, P. et al. Outcomes of renal transplant in patients with anti-complement factor H antibody-associated hemolytic uremic syndrome. Pediatr. Transplant. 18, E134–E139 (2014).
Midtvedt, K., Bitter, J. & Dorje, C. Belatacept as immunosuppression in patient with recurrence of HUS after renal transplantation. Transplantation 87, 1901–1903 (2009).
Noris, M. et al. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood 124, 1715–1726 (2014).
Cochat, P. & Rumsby, G. Primary hyperoxaluria. N. Engl. J. Med. 369, 649–658 (2013).
Cochat, P. et al. Primary hyperoxaluria Type 1: indications for screening and guidance for diagnosis and treatment. Nephrol. Dial. Transplant. 27, 1729–1736 (2012).
Filler, G. & Hoppe, B. Combined liver-kidney transplantation for hyperoxaluria type II? Pediatr. Transplant. 18, 237–239 (2014).
Harambat, J., Fargue, S., Bacchetta, J., Acquaviva, C. & Cochat, P. Primary hyperoxaluria. Int. J. Nephrol. 2011, 864580 (2011).
Naderi, G., Latif, A., Tabassomi, F. & Esfahani, S. T. Failure of isolated kidney transplantation in a pediatric patient with primary hyperoxaluria type 2. Pediatr. Transplant. 18, E69–E73 (2014).
Harambat, J. et al. Characteristics and outcomes of children with primary oxalosis requiring renal replacement therapy. Clin. J. Am. Soc. Nephrol. 7, 458–465 (2012).
Brinkert, F. et al. Transplantation procedures in children with primary hyperoxaluria type 1: outcome and longitudinal growth. Transplantation 87, 1415–1421 (2009).
Perera, M. T. et al. Pre-emptive liver transplantation for primary hyperoxaluria (PHI) arrests long-term renal function deterioration. Nephrol. Dial. Transplant. 26, 354–359 (2011).
Jamieson, N. V. & the European PH1 Transplantation Study Group. A 20-year experience of combined liver/kidney transplantation for primary hyperoxaluria (PH1): the European PH1 transplant registry experience 1984–2004. Am. J. Nephrol. 25, 282–289 (2005).
Cibrik, D. M., Kaplan, B., Arndorfer, J. A. & Meier-Kriesche, H. U. Renal allograft survival in patients with oxalosis. Transplantation 74, 707–710 (2002).
Millan, M. T. et al. One hundred percent patient and kidney allograft survival with simultaneous liver and kidney transplantation in infants with primary hyperoxaluria: a single-center experience. Transplantation 76, 1458–1463 (2003).
Ellis, S. R., Hulton, S. A., McKiernan, P. J., de Ville de Goyet, J. & Kelly, D. A. Combined liver-kidney transplantation for primary hyperoxaluria type 1 in young children. Nephrol. Dial. Transplant. 16, 348–354 (2001).
Bergstralh, E. J. et al. Transplantation outcomes in primary hyperoxaluria. Am. J. Transplant. 10, 2493–2501 (2010).
Malla, I. et al. Two-step transplantation for primary hyperoxaluria: cadaveric liver followed by living donor related kidney transplantation. Pediatr. Transplant. 13, 782–784 (2009).
Lai, C. et al. Inhibition of glycolate oxidase (HAO1) with dicer-substrate siRNAs as a substrate reduction therapy for primary hyperoxaluria Type I [online], (2014).
Bollee, G. et al. Adenine phosphoribosyltransferase deficiency. Clin. J. Am. Soc. Nephrol. 7, 1521–1527 (2012).
Harambat, J., Bollee, G., Daudon, M., Ceballos-Picot, I. & Bensman, A. Adenine phosphoribosyltransferase deficiency in children. Pediatr. Nephrol. 27, 571–579 (2012).
Gagne, E. R., Deland, E., Daudon, M., Noel, L. H. & Nawar, T. Chronic renal failure secondary to 2, 8-dihydroxyadenine deposition: the first report of recurrence in a kidney transplant. Am. J. Kidney Dis. 24, 104–107 (1994).
Cassidy, M. J., McCulloch, T., Fairbanks, L. D. & Simmonds, H. A. Diagnosis of adenine phosphoribosyltransferase deficiency as the underlying cause of renal failure in a renal transplant recipient. Nephrol. Dial. Transplant. 19, 736–738 (2004).
Kaartinen, K. et al. Adenine phosphoribosyltransferase deficiency as a rare cause of renal allograft dysfunction. J. Am. Soc. Nephrol. 25, 671–674 (2014).
Stratta, P. et al. Decreased kidney function and crystal deposition in the tubules after kidney transplant. Am. J. Kidney Dis. 56, 585–590 (2010).
Nasr, S. H. et al. Crystalline nephropathy due to 2, 8-dihydroxyadeninuria: an under-recognized cause of irreversible renal failure. Nephrol. Dial. Transplant. 25, 1909–1915 (2010).
Zaidan, M. et al. Recurrent 2, 8-dihydroxyadenine nephropathy: a rare but preventable cause of renal allograft failure. Am. J. Transplant. 14, 2623–2632 (2014).
Bollee, G. et al. Phenotype and genotype characterization of adenine phosphoribosyltransferase deficiency. J. Am. Soc. Nephrol. 21, 679–688 (2010).
Hogg, R. J. Idiopathic immunoglobulin A nephropathy in children and adolescents. Pediatr. Nephrol. 25, 823–829 (2010).
Ortiz, F. et al. IgA nephropathy recurs early in the graft when assessed by protocol biopsy. Nephrol. Dial. Transplant. 27, 2553–2558 (2012).
Coppo, R. et al. Predictors of outcome in Henoch-Schonlein nephritis in children and adults. Am. J. Kidney Dis. 47, 993–1003 (2006).
Narchi, H. Risk of long term renal impairment and duration of follow up recommended for Henoch-Schonlein purpura with normal or minimal urinary findings: a systematic review. Arch. Dis. Child. 90, 916–920 (2005).
Coppo, R. et al. Serological and genetic factors in early recurrence of IgA nephropathy after renal transplantation. Clin. Transplant. 21, 728–737 (2007).
Kanaan, N. et al. Recurrence and graft loss after kidney transplantation for henoch-schonlein purpura nephritis: a multicenter analysis. Clin. J. Am. Soc. Nephrol. 6, 1768–1772 (2011).
Moroni, G. et al. Renal transplantation in adults with Henoch-Schonlein purpura: long-term outcome. Nephrol. Dial. Transplant. 23, 3010–3016 (2008).
Oliveira, C. S. et al. Renal transplantation in lupus nephritis: a Brazilian cohort. Lupus 21, 570–574 (2012).
Moroni, G. et al. The long-term prognosis of renal transplantation in patients with lupus nephritis. Am. J. Kidney Dis 45, 903–911 (2005).
Yu, T. M. et al. Impact of recurrent lupus nephritis on lupus kidney transplantation: a 20-year single center experience. Clin. Rheumatol. 31, 705–710 (2012).
Burgos, P. I. et al. Risk factors and impact of recurrent lupus nephritis in patients with systemic lupus erythematosus undergoing renal transplantation: date from a single US institution. Arthritis Rheum. 60, 2757–2766 (2009).
Norby, G. E. et al. Recurrent lupus nephritis after kidney transplantation: a surveillance biopsy study. Ann. Rheum. Dis. 69, 1484–1487 (2010).
Stone, J. H., Amend, W. J. & Criswell, L. A. Antiphospholipid antibody syndrome in renal transplantation: occurrence of clinical events in 96 consecutive patients with systemic lupus erythematosus. Am. J. Kidney Dis. 34, 1040–1047 (1999).
Gonzalez-Pulido, C., Croca, S., Abrol, E. & Isenberg, D. A. Long-term activity index after renal failure in a cohort of 32 patients with lupus nephritis. Clin. Exp. Rheumatol. 32, 301–307 (2014).
Yu, T. M. et al. Renal outcome and evolution of disease activity in Chinese lupus patients after renal transplantation. Lupus 17, 687–694 (2008).
Friedman, G. S. et al. Hypercoagulable states in renal transplant candidates: impact of anticoagulation upon incidence of renal allograft thrombosis. Transplantation 72, 1073–1078 (2001).
Morrissey, P. E. et al. Management of thrombophilia in renal transplant patients. Am. J. Transplant. 2, 872–876 (2002).
Barbour, T. D. et al. Antiphospholipid syndrome in renal transplantation. Nephrology (Carlton) 19, 177–185 (2014).
Falkeis, C. et al. Kidney transplantation in patients suffering from hereditary complete complement C4 deficiency. Transpl. Int. 20, 1044–1049 (2007).
Little, M. A. et al. Renal transplantation in systemic vasculitis: when is it safe? Nephrol. Dial. Transplant. 24, 3219–3125 (2009).
Booth, A. D. et al. Outcome of ANCA-associated renal vasculitis: a 5year retrospective study. Am. J. Kidney Dis. 41, 776–784 (2003).
Marco, H. et al. Long-term outcome of antineutrophil cytoplasmic antibody-associated small vessel vasculitis after renal transplantation. Clin. Transplant. 27, 338–347 (2013).
Elmedhem, A., Adu, D. & Savage, C. O. Relapse rate and outcome of ANCA-associated small vessel vasculitis after transplantation. Nephrol. Dial. Transplant. 18, 1001–1004 (2003).
Murakami, C., Manoharan, P., Carter-Monroe, N. & Geetha, D. Rituximab for remission induction in recurrent ANCA-associated glomerulonephritis postkidney transplant. Transpl. Int. 26, 1225–1231 (2013).
Geetha, D., Seo, P., Specks, U. & Fervenza, F. C. Successful induction of remission with rituximab for relapse of ANCA-associated vasculitis post-kidney transplant: report of two cases. Am. J. Transplant. 7, 2821–2825 (2007).
Van Stralen, K. J. et al. Improvement in the renal prognosis in nephropathic cystinosis. Clin. J. Am. Soc. Nephrol. 6, 2485–2491 (2011).
Ojo, A. et al. Excellent outcome of renal transplantation in patients with Fabry's disease. Transplantation 69, 2337–2339 (2000).
Luan, F. L. & Samaniego, M. Transplantation in diabetic kidney failure patients: modalities, outcomes, and clinical management. Semin. Dial. 23, 198–205 (2010).
Nath, J. et al. Sickle cell and renal transplant: a national survey and literature review. Exp. Clin. Transplant. 10, 1–7 (2012).
Vargas, F., Gedalia, A., Craver, R. D. & Matti Vehaskari, V. Recurrence of granulomatous interstitial nephritis in transplanted kidney. Pediatr. Transplant. 14, e54–e57 (2010).
Author information
Authors and Affiliations
Contributions
J.B. and P.C. contributed equally to researching data for the article and discussions of the article's content. J.B. wrote the manuscript, and J.B. and P.C. reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bacchetta, J., Cochat, P. Primary disease recurrence—effects on paediatric renal transplantation outcomes. Nat Rev Nephrol 11, 371–384 (2015). https://doi.org/10.1038/nrneph.2015.54
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2015.54
This article is cited by
-
Precise clinicopathologic findings for application of genetic testing in pediatric kidney transplant recipients with focal segmental glomerulosclerosis/steroid-resistant nephrotic syndrome
Pediatric Nephrology (2023)
-
Management and outcomes in children with lupus nephritis in the developing countries
Pediatric Nephrology (2023)
-
How common is chronic kidney disease in children with lupus nephritis?
Pediatric Nephrology (2023)
-
Hemolytic uremic syndrome in a developing country: Consensus guidelines
Pediatric Nephrology (2019)
-
LDL-apheresis-induced remission of focal segmental glomerulosclerosis recurrence in pediatric renal transplant recipients
Pediatric Nephrology (2019)