Key Points
-
Heterozygous mutations in the gene encoding the transcription factor HNF1B result in a multi-system disorder and are the most common known monogenic cause of developmental renal disease
-
HNF1B mutations comprise base substitutions, small insertions–deletions, or whole-gene deletions; however, no evidence exists for a genotype–phenotype correlation
-
HNF1B-associated disease exhibits autosomal dominant inheritance; however, mutations and whole-gene deletions can occur spontaneously so family history of renal disease or diabetes mellitus may be absent
-
HNF1B is expressed in multiple fetal tissues and has an important role during several stages of nephrogenesis, including ureteric bud branching and tubular development
-
HNF1B-associated renal phenotypes are variable and include isolated bilateral hyperechogenic kidneys on prenatal ultrasonography; cysts; hypoplasia; single, horseshoe and duplex kidneys; collecting system abnormalities; bilateral hydronephrosis; and hyperuricaemic nephropathy
-
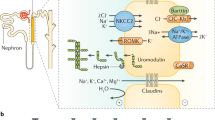
Electrolyte abnormalities include hypomagnesaemia and hyperuricaemia; extra-renal phenotypic features include early-onset diabetes mellitus, pancreatic hypoplasia, genital tract malformations and abnormal liver function test results
Abstract
Heterozygous mutations in the gene that encodes the transcription factor hepatocyte nuclear factor 1β (HNF1B) represent the most common known monogenic cause of developmental kidney disease. Renal cysts are the most frequently detected feature of HNF1B-associated kidney disease; however, other structural abnormalities, including single kidneys and renal hypoplasia, and electrolyte abnormalities can also occur. Extra-renal phenotypes might also be observed; consequently, HNF1B-associated disease is considered a multi-system disorder. Other clinical features include early-onset diabetes mellitus, pancreatic hypoplasia, genital tract malformations, abnormal liver function and early-onset gout. Heterozygous mutations in the coding region or splice sites of HNF1B, and complete gene deletion, each account for ∼50% of all cases of HNF1B-associated disease, respectively, and often arise spontaneously. There is no clear genotype–phenotype correlation, consistent with haploinsufficiency as the disease mechanism. Data from animal models suggest that HNF1B has an important function during several stages of nephrogenesis; however, the precise signalling pathways remain to be elucidated. This Review discusses the genetics and molecular pathways that lead to disease development, summarizes the reported renal and extra-renal phenotypes, and identifies areas for future research in HNF1B-associated disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Madariaga, L. et al. Severe prenatal renal anomalies associated with mutations in HNF1B or PAX2 genes. Clin. J. Am. Soc. Nephrol. 8, 1179–1187 (2013).
Thomas, R. et al. HNF1B and PAX2 mutations are a common cause of renal hypodysplasia in the CKiD cohort. Pediatr. Nephrol. 26, 897–903 (2011).
Weber, S. et al. Prevalence of mutations in renal developmental genes in children with renal hypodysplasia: results of the ESCAPE study. J. Am. Soc. Nephrol. 17, 2864–2870 (2006).
Heidet, L. et al. Spectrum of HNF1B mutations in a large cohort of patients who harbor renal diseases. Clin. J. Am. Soc. Nephrol. 5, 1079–1090 (2010).
Bingham, C. et al. Mutations in the hepatocyte nuclear factor-1β gene are associated with familial hypoplastic glomerulocystic kidney disease. Am. J. Hum. Genet. 68, 219–224 (2001).
Horikawa, Y. et al. Mutation in hepatocyte nuclear factor-1β gene (TCF2) associated with MODY. Nat. Genet. 17, 384–385 (1997).
Iwasaki, N. et al. Liver and kidney function in Japanese patients with maturity-onset diabetes of the young. Diabetes Care 21, 2144–2148 (1998).
Nishigori, H. et al. Frameshift mutation, A263fsinsGG, in the hepatocyte nuclear factor-1β gene associated with diabetes and renal dysfunction. Diabetes 47, 1354–1355 (1998).
Bellanne-Chantelot, C. et al. Clinical spectrum associated with hepatocyte nuclear factor-1β mutations. Ann. Intern. Med. 140, 510–517 (2004).
Haldorsen, I. S. et al. Lack of pancreatic body and tail in HNF1B mutation carriers. Diabet. Med. 25, 782–787 (2008).
Lindner, T. H. et al. A novel syndrome of diabetes mellitus, renal dysfunction and genital malformation associated with a partial deletion of the pseudo-POU domain of hepatocyte nuclear factor-1β. Hum. Mol. Genet. 8, 2001–2008 (1999).
Montoli, A. et al. Renal cysts and diabetes syndrome linked to mutations of the hepatocyte nuclear factor-1β gene: description of a new family with associated liver involvement. Am. J. Kidney Dis. 40, 397–402 (2002).
Adalat, S. et al. HNF1B mutations associate with hypomagnesemia and renal magnesium wasting. J. Am. Soc. Nephrol. 20, 1123–1131 (2009).
Bingham, C. et al. Atypical familial juvenile hyperuricemic nephropathy associated with a hepatocyte nuclear factor-1β gene mutation. Kidney Int. 63, 1645–1651 (2003).
Tattersall, R. B. Mild familial diabetes with dominant inheritance. Q. J. Med. 43, 339–357 (1974).
Frayling, T. M. et al. Mutations in the hepatocyte nuclear factor-1α gene are a common cause of maturity-onset diabetes of the young in the U.K. Diabetes 46, 720–725 (1997).
Mendel, D. B., Hansen, L. P., Graves, M. K., Conley, P. B. & Crabtree, G. R. HNF-1α and HNF-1β (vHNF-1) share dimerization and homeo domains, but not activation domains, and form heterodimers in vitro. Genes Dev. 5, 1042–1056 (1991).
Ulinski, T. et al. Renal phenotypes related to hepatocyte nuclear factor-1β (TCF2) mutations in a pediatric cohort. J. Am. Soc. Nephrol. 17, 497–503 (2006).
Decramer, S. et al. Anomalies of the TCF2 gene are the main cause of fetal bilateral hyperechogenic kidneys. J. Am. Soc. Nephrol. 18, 923–933 (2007).
Edghill, E. L. et al. Hepatocyte nuclear factor-1β gene deletions-—a common cause of renal disease. Nephrol. Dial. Transplant. 23, 627–635 (2008).
Nakayama, M. et al. HNF1B alterations associated with congenital anomalies of the kidney and urinary tract. Pediatr. Nephrol. 25, 1073–1079 (2010).
Faguer, S. et al. Diagnosis, management, and prognosis of HNF1B nephropathy in adulthood. Kidney Int. 80, 768–776 (2011).
Raile, K. et al. Expanded clinical spectrum in hepatocyte nuclear factor 1b-maturity-onset diabetes of the young. J. Clin. Endocrinol. Metab. 94, 2658–2664 (2009).
Oram, R. A. et al. Mutations in the hepatocyte nuclear factor-1β (HNF1B) gene are common with combined uterine and renal malformations but are not found with isolated uterine malformations. Am. J. Obstet. Gynecol. 203, 364.e1–364.e5 (2010).
Chen, Y. Z. et al. Systematic review of TCF2 anomalies in renal cysts and diabetes syndrome/maturity onset diabetes of the young type 5. Chin. Med. J. (Eng.) 123, 3326–3333 (2010).
Edghill, E. L., Bingham, C., Ellard, S. & Hattersley, A. T. Mutations in hepatocyte nuclear factor-1β and their related phenotypes. J. Med. Genet. 43, 84–90 (2006).
Bellanne-Chantelot, C. et al. Large genomic rearrangements in the hepatocyte nuclear factor-1β (TCF2) gene are the most frequent cause of maturity-onset diabetes of the young type 5. Diabetes 54, 3126–3132 (2005).
Mefford, H. C. et al. Recurrent reciprocal genomic rearrangements of 17q12 are associated with renal disease, diabetes, and epilepsy. Am. J. Hum. Genet. 81, 1057–1069 (2007).
Ellard, S. et al. Improved genetic testing for monogenic diabetes using targeted next-generation sequencing. Diabetologia 56, 1958–1963 (2013).
Bingham, C. et al. Abnormal nephron development associated with a frameshift mutation in the transcription factor hepatocyte nuclear factor-1β. Kidney Int. 57, 898–907 (2000).
Sun, Z. & Hopkins, N. vhnf1, the MODY5 and familial GCKD-associated gene, regulates regional specification of the zebrafish gut, pronephros, and hindbrain. Genes Dev. 15, 3217–3229 (2001).
Bohn, S. et al. Distinct molecular and morphogenetic properties of mutations in the human HNF1β gene that lead to defective kidney development. J. Am. Soc. Nephrol. 14, 2033–2041 (2003).
Barbacci, E. et al. HNF1β/TCF2 mutations impair transactivation potential through altered co-regulator recruitment. Hum. Mol. Genet. 13, 3139–3149 (2004).
Harries, L. W., Ellard, S., Jones, R. W., Hattersley, A. T. & Bingham, C. Abnormal splicing of hepatocyte nuclear factor-1β in the renal cysts and diabetes syndrome. Diabetologia 47, 937–942 (2004).
Harries, L. W., Bingham, C., Bellanne-Chantelot, C., Hattersley, A. T. & Ellard, S. The position of premature termination codons in the hepatocyte nuclear factor-1β gene determines susceptibility to nonsense-mediated decay. Hum. Genet. 118, 214–224 (2005).
Faguer, S. et al. Expression of renal cystic genes in patients with HNF1B mutations. Nephron Clin. Pract 120, c71–78 (2012).
Barbacci, E. et al. Variant hepatocyte nuclear factor 1 is required for visceral endoderm specification. Development 126, 4795–4805 (1999).
Cereghini, S., Ott, M. O., Power, S. & Maury, M. Expression patterns of vHNF1 and HNF1 homeoproteins in early postimplantation embryos suggest distinct and sequential developmental roles. Development 116, 783–797 (1992).
Bingham, C. & Hattersley, A. T. Renal cysts and diabetes syndrome resulting from mutations in hepatocyte nuclear factor-1β. Nephrol. Dial. Transplant. 19, 2703–2708 (2004).
Dressler, G. R. Advances in early kidney specification, development and patterning. Development 136, 3863–3874 (2009).
Dressler, G. R. The cellular basis of kidney development. Annu. Rec. Cell Dev. Biol. 22, 509–529 (2006).
Kolatsi-Joannou, M. et al. Hepatocyte nuclear factor-1β: a new kindred with renal cysts and diabetes and gene expression in normal human development. J. Am. Soc. Nephrol. 12, 2175–2180 (2001).
Lokmane, L., Heliot, C., Garcia-Villalba, P., Fabre, M. & Cereghini, S. vHNF1 functions in distinct regulatory circuits to control ureteric bud branching and early nephrogenesis. Development 137, 347–357 (2010).
Carroll, T. J., Park, J. S., Hayashi, S., Majumdar, A. & McMahon, A. P. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev. Cell 9, 283–292 (2005).
Gresh, L. et al. A transcriptional network in polycystic kidney disease. EMBO J. 23, 1657–1668 (2004).
Hiesberger, T. et al. Mutation of hepatocyte nuclear factor-1β inhibits Pkhd1 gene expression and produces renal cysts in mice. J. Clin. Invest. 113, 814–825 (2004).
Massa, F. et al. Hepatocyte nuclear factor 1β controls nephron tubular development. Development 140, 886–896 (2013).
Cheng, H. T. et al. Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development 134, 801–811 (2007).
Caiulo, V. A. et al. Ultrasound mass screening for congenital anomalies of the kidney and urinary tract. Pediatr. Nephrol. 27, 949–953 (2012).
North American Pediatric Renal Trials and Collaborative Studies: NAPRTCS 2011 Annual Report, Rockville, MD, The EMMES Corporation, 2011 (2011).
Edghill, E. L. et al. HNF1B deletions in patients with young-onset diabetes but no known renal disease. Diabet. Med. 30, 114–117 (2013).
Avni, F. E. & Hall, M. Renal cystic diseases in children: new concepts. Pediatr. Radiol. 40, 939–946 (2010).
Murray, P. J. et al. Whole gene deletion of the hepatocyte nuclear factor-1β gene in a patient with the prune-belly syndrome. Nephrol. Dial. Transplant. 23, 2412–2415 (2008).
Haeri, S. et al. Deletion of hepatocyte nuclear factor-1-β in an infant with prune belly syndrome. Am. J. Perinatol. 27, 559–563 (2010).
Granberg, C. F. et al. Genetic basis of prune belly syndrome: screening for HNF1β gene. J. Urol. 187, 272–278 (2012).
Mache, C. J., Preisegger, K. H., Kopp, S., Ratschek, M. & Ring, E. De novo HNF-1β gene mutation in familial hypoplastic glomerulocystic kidney disease. Pediatr. Nephrol. 17, 1021–1026 (2002).
Sagen, J. V., Bostad, L., Njolstad, P. R. & Sovik, O. Enlarged nephrons and severe nondiabetic nephropathy in hepatocyte nuclear factor-1β (HNF-1β) mutation carriers. Kidney Int. 64, 793–800 (2003).
Bingham, C. et al. Solitary functioning kidney and diverse genital tract malformations associated with hepatocyte nuclear factor-1β mutations. Kidney Int. 61, 1243–1251 (2002).
Rebouissou, S. et al. Germline hepatocyte nuclear factor 1alpha and 1beta mutations in renal cell carcinomas. Hum. Mol. Genet. 14, 603–614 (2005).
Tsuchiya, A. et al. Expression profiling in ovarian clear cell carcinoma: identification of hepatocyte nuclear factor-1β as a molecular marker and a possible molecular target for therapy of ovarian clear cell carcinoma. Am. J. Pathol. 163, 2503–2512 (2003).
Spurdle, A. B. et al. Genome-wide association study identifies a common variant associated with risk of endometrial cancer. Nat. Genet. 43, 451–454 (2011).
Gudmundsson, J. et al. Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat. Genet. 39, 977–983 (2007).
Thomas, G. et al. Multiple loci identified in a genome-wide association study of prostate cancer. Nat. Genet. 40, 310–315 (2008).
Sun, J. et al. Evidence for two independent prostate cancer risk-associated loci in the HNF1B gene at 17q12. Nat. Genet. 40, 1153–1155 (2008).
Ferre, S., Veenstra, G. J., Bouwmeester, R., Hoenderop, J. G. & Bindels, R. J. HNF-1B specifically regulates the transcription of the γa-subunit of the Na+/K+-ATPase. Biochem. Biophys. Res. Commun. 404, 284–290 (2011).
Meij, I. C. et al. Dominant isolated renal magnesium loss is caused by misrouting of the Na(+), K(+)-ATPase γ-subunit. Nat. Genet. 26, 265–266 (2000).
Hart, T. C. et al. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J. Med. Genet. 39, 882–892 (2002).
Zuber, J. et al. HNF1B-related diabetes triggered by renal transplantation. Nat. Rev. Nephrol. 5, 480–484 (2009).
Halbritter, J. et al. Successful simultaneous pancreas kidney transplantation in maturity-onset diabetes of the young type 5. Transplantation 92, e45–e47 (2011).
Poitou, C. et al. Maturity onset diabetes of the young: clinical characteristics and outcome after kidney and pancreas transplantation in MODY3 and RCAD patients: a single center experience. Transplant. Int. 25, 564–572 (2012).
Maestro, M. A. et al. Hnf6 and Tcf2 (MODY5) are linked in a gene network operating in a precursor cell domain of the embryonic pancreas. Hum. Mol. Genet. 12, 3307–3314 (2003).
Haumaitre, C. et al. Lack of TCF2/vHNF1 in mice leads to pancreas agenesis. Proc. Natl Acad. Sci. USA. 102, 1490–1495 (2005).
Yorifuji, T. et al. Neonatal diabetes mellitus and neonatal polycystic, dysplastic kidneys: Phenotypically discordant recurrence of a mutation in the hepatocyte nuclear factor-1β gene due to germline mosaicism. J. Clin. Endocrinol. Metab. 89, 2905–2908 (2004).
Edghill, E. L. et al. Hepatocyte nuclear factor-1β mutations cause neonatal diabetes and intrauterine growth retardation: support for a critical role of HNF-1β in human pancreatic development. Diabet. Med. 23, 1301–1306 (2006).
Pearson, E. R. et al. Contrasting diabetes phenotypes associated with hepatocyte nuclear factor-1α and -1β mutations. Diabetes Care 27, 1102–1107 (2004).
Pearson, E. R. et al. Contrasting diabetes phenotypes associated with hepatocyte nuclear factor-1α and -1β mutations. Diabetes Care 27, 1102–1107 (2004).
Brackenridge, A. et al. Contrasting insulin sensitivity of endogenous glucose production rate in subjects with hepatocyte nuclear factor-1β and -1α mutations. Diabetes 55, 405–411 (2006).
Body-Bechou, D. et al. TCF2/HNF-1β mutations: 3 cases of fetal severe pancreatic agenesis or hypoplasia and multicystic renal dysplasia. Prenat. Diagn. 34, 90–93 (2014).
Tjora, E. et al. Exocrine pancreatic function in hepatocyte nuclear factor 1β-maturity-onset diabetes of the young (HNF1B-MODY) is only moderately reduced: compensatory hypersecretion from a hypoplastic pancreas. Diabet. Med. 30, 946–955 (2013).
Iwasaki, N. et al. Splice site mutation in the hepatocyte nuclear factor-1 β gene, IVS2nt + 1G > A, associated with maturity-onset diabetes of the young, renal dysplasia and bicornuate uterus. Diabetologia 44, 387–388 (2001).
Bernardini, L. et al. Recurrent microdeletion at 17q12 as a cause of Mayer-Rokitansky-Kuster-Hauser (MRKH) syndrome: two case reports. Orphanet J. Rare Dis. 4, 25 (2009).
Ledig, S. et al. Recurrent aberrations identified by array-CGH in patients with Mayer-Rokitansky-Kuster-Hauser syndrome. Fertil. Steril. 95, 1589–1594 (2011).
Kitanaka, S., Miki, Y., Hayashi, Y. & Igarashi, T. Promoter-specific repression of hepatocyte nuclear factor (HNF)-1β and HNF-1α transcriptional activity by an HNF-1β missense mutant associated with Type 5 maturity-onset diabetes of the young with hepatic and biliary manifestations. J. Clin. Endocrinol. Metab. 89, 1369–1378 (2004).
Beckers, D., Bellanne-Chantelot, C. & Maes, M. Neonatal cholestatic jaundice as the first symptom of a mutation in the hepatocyte nuclear factor-1β gene (HNF-1β). J. Pediatr. 150, 313–314 (2007).
Coffinier, C. et al. Bile system morphogenesis defects and liver dysfunction upon targeted deletion of HNF1β. Development 129, 1829–1838 (2002).
Roelandt, P. et al. HNF1B deficiency causes ciliary defects in human cholangiocytes. Hepatology 56, 1178–1181 (2012).
Moreno-De-Luca, D. et al. Deletion 17q12 is a recurrent copy number variant that confers high risk of autism and schizophrenia. Am. J. Hum. Genet. 87, 618–630 (2010).
Nagamani, S. C. et al. Clinical spectrum associated with recurrent genomic rearrangements in chromosome 17q12. Eur. J. Hum. Genet. 18, 278–284 (2010).
Loirat, C. et al. Autism in three patients with cystic or hyperechogenic kidneys and chromosome 17q12 deletion. Nephrol. Dial. Transplant. 25, 3430–3433 (2010).
Ferre, S. et al. Early development of hyperparathyroidism due to loss of PTH transcriptional repression in patients with HNF1β mutations? J. Clin. Endocrinol. Metab. 98, 4089–4096 (2013).
Faguer, S. et al. The HNF1B score is a simple tool to select patients for HNF1B gene analysis. Kidney Int. 86, 1007–1015 (2014).
Kohl, S. et al. Mild recessive mutations in six Fraser syndrome-related genes cause isolated congenital anomalies of the kidney and urinary tract. J. Am. Soc. Nephrol. 25, 1917–1922 (2014).
Stenson, P. D. et al. Human Gene Mutation Database (HGMD): 2003 update. Hum. Mutat. 21, 577–581 (2003).
Barratt, J., Topham, P. & Harris, K. Oxford Desk Reference: Nephrology (Oxford University Press, 2009).
Kerecuk, L., Schreuder, M. F. & Woolf, A. S. Renal tract malformations: perspectives for nephrologists. Nat. Clin. Pract. Nephrol. 4, 312–325 (2008).
Acknowledgements
The authors' research is supported by a Medical Research Council Clinical Training Fellowship (MR/J011630/1) to R.L.C., a National Institute for Health Research Senior Investigator award (NF-SI-0611-10219) to A.T.H. and a Wellcome Trust Senior Investigator award (098,395/Z/12/Z) to S.E. and A.T.H. The authors thank Kevin Colclough (Exeter Molecular Genetics Laboratory, UK) for his assistance in the preparation of Figure 2.
Author information
Authors and Affiliations
Contributions
R.L.C. and A.J.H. researched the data for the article. R.L.C., A.T.H., S.E. and C.B provided substantial contribution to discussions of the content. R.L.C. and A.J.H. contributed equally to writing the article. All authors contributed to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
HNF1B mutations and protein effects, as listed in the Human Gene Mutation Database (PDF 516 kb)
Rights and permissions
About this article
Cite this article
Clissold, R., Hamilton, A., Hattersley, A. et al. HNF1B-associated renal and extra-renal disease—an expanding clinical spectrum. Nat Rev Nephrol 11, 102–112 (2015). https://doi.org/10.1038/nrneph.2014.232
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2014.232
This article is cited by
-
Maturity-onset diabetes of the young secondary to HNF1B variants (HNF1B-MODY): a series of 10 patients from a single diabetes center
Diabetology & Metabolic Syndrome (2023)
-
Reversed cortico-medullary differentiation in the fetal and neonatal kidneys: an indicator of poor prognosis?
Pediatric Radiology (2023)
-
The genetic basis of congenital anomalies of the kidney and urinary tract
Pediatric Nephrology (2022)
-
Urinary tract endometriosis masquerading as a renal mass in hepatocyte nuclear factor-1 beta gene mutation
International Urology and Nephrology (2022)
-
Elevated level of lysophosphatidic acid among patients with HNF1B mutations and its role in RCAD syndrome: a multiomic study
Metabolomics (2022)