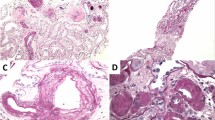
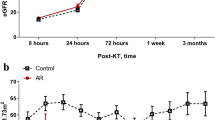
Key Points
-
Biomarkers can be classified as prognostic or predictive biomarkers, and surrogate end points
-
Sensitivity, specificity, positive and negative predictive values, and receiver operating characteristic curves are important measures used to assess the clinical utility of a biomarker
-
Noninvasive candidate biomarkers of early or late graft rejection found in peripheral blood and urine include mRNA transcripts, lymphocyte phenotype markers, chemokines, microRNAs, and donor specific antibodies
-
Robust validation studies and assay standardization are required before biomarkers can be used in patient care
-
Biomarker validation will be challenging, given the interindividual variation in transplant recipients
Abstract
The immune management of organ transplant recipients is imperfect. Beyond general dosing guidelines for immunosuppressive agents and clinical diagnostic tests for rejection or infection, there are few objective tools to determine the aggregate status of a patient's alloimmune response or protective immune capacity. The lack of prognostic precision significantly contributes to patient morbidity and reduces long-term allograft survival after kidney transplantation. Noninvasive biomarkers that could serve as predictive tools or surrogate end points for rejection might help clinicians individualize immunosuppression and allow for early intervention, ideally prior to clinically evident organ dysfunction. Although the growing understanding of organ rejection has provided numerous candidate biomarkers, none has been confirmed in robust validation studies as sufficiently useful to guide clinical practice independent of traditional clinical methods. In this Review, the general characteristics of biomarkers and surrogate end points; current biomarkers under active clinical investigation; and the prominent barriers to the translation of biomarkers into clinical practice are discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kaneku, H. K. & Terasaki, P. I. Thirty year trend in kidney transplants: UCLA and UNOS Renal Transplant Registry. Clin. Transpl. 1–27 (2006).
Biomarker definitions workgroup. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 69, 89–95 (2001).
Lachenbruch, P. A., Rosenberg, A. S., Bonvini, E., Cavaille-Coll, M. W. & Colvin, R. B. Biomarkers and surrogate endpoints in renal transplantation: present status and considerations for clinical trial design. Am. J. Transplant. 4, 451–457 (2004).
Singh, N. et al. Pretransplant donor-specific antibodies detected by single-antigen bead flow cytometry are associated with inferior kidney transplant outcomes. Transplantation 90, 1079–1084 (2010).
Racusen, L. C. et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 55, 713–723 (1999).
Solez, K. et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am. J. Transplant. 8, 753–760 (2008).
Thaunat, O., Legendre, C., Morelon, E., Kreis, H. & Mamzer-Bruneel, M. F. To biopsy or not to biopsy? Should we screen the histology of stable renal grafts? Transplantation 84, 671–676 (2007).
Kirk, A. D. et al. Clinically stable human renal allografts contain histological and RNA-based findings that correlate with deteriorating graft function. Transplantation 68, 1578–1582 (1999).
Rush, D. N., Henry, S. F., Jeffery, J. R., Schroeder, T. J. & Gough, J. Histological findings in early routine biopsies of stable renal allograft recipients. Transplantation 57, 208–211 (1994).
Rush, D. et al. Lack of benefit of early protocol biopsies in renal transplant patients receiving TAC and MMF: a randomized study. Am. J. Transplant. 7, 2538–2545 (2007).
Mueller, T. F. & Mas, V. R. Microarray applications in nephrology with special focus on transplantation. J. Nephrol. 25, 589–602 (2012).
Mas, V. R., Dumur, C. I., Scian, M. J., Gehrau, R. C. & Maluf, D. G. MicroRNAs as biomarkers in solid organ transplantation. Am. J. Transplant. 13, 11–19 (2013).
Sigdel, T. K. & Sarwal, M. M. Recent advances in biomarker discovery in solid organ transplant by proteomics. Expert Rev. Proteomics 8, 705–715 (2011).
Chattopadhyay, P. K. & Roederer, M. Cytometry: today's technology and tomorrow's horizons. Methods 57, 251–258 (2012).
Augustine, J. J. & Hricik, D. E. T-cell immune monitoring by the ELISPOT assay for interferon γ. Clin. Chim. Acta 413, 1359–1363 (2012).
Barry, M. & Bleackley, R. C. Cytotoxic T lymphocytes: all roads lead to death. Nat. Rev. Immunol. 2, 401–409 (2002).
Cornell, L. D., Smith, R. N. & Colvin, R. B. Kidney transplantation: mechanisms of rejection and acceptance. Annu. Rev. Pathol. 3, 189–220 (2008).
Vasconcellos, L. M. et al. Cytotoxic lymphocyte gene expression in peripheral blood leukocytes correlates with rejecting renal allografts. Transplantation 66, 562–566 (1998).
Gibson, I. W. et al. Peritubular capillaritis in renal allografts: prevalence, scoring system, reproducibility and clinicopathological correlates. Am. J. Transplant. 8, 819–825 (2008).
Marcussen, N., Olsen, T. S., Benediktsson, H., Racusen, L. & Solez, K. Reproducibility of the Banff classification of renal allograft pathology. Inter- and intraobserver variation. Transplantation 60, 1083–1089 (1995).
Aquino-Dias, E. C. et al. Non-invasive diagnosis of acute rejection in kidney transplants with delayed graft function. Kidney Int. 73, 877–884 (2008).
Netto, M. V. et al. Granzyme, B., Fas-ligand and perforin expression during acute cellular rejection episodes after kidney transplantation: comparison between blood and renal aspirates. Transplant. Proc. 34, 476–478 (2002).
Sabek, O., Dorak, M. T., Kotb, M., Gaber, A. O. & Gaber, L. Quantitative detection of T-cell activation markers by real-time PCR in renal transplant rejection and correlation with histopathologic evaluation. Transplantation 74, 701–707 (2002).
Graziotto, R. et al. Perforin, granzyme B, and Fas ligand for molecular diagnosis of acute renal-allograft rejection: analyses on serial biopsies suggest methodological issues. Transplantation 81, 1125–1132 (2006).
Heidt, S. et al. Peripheral blood sampling for the detection of allograft rejection: biomarker identification and validation. Transplantation 92, 1–9 (2011).
Kirk, A. D., Bollinger, R. R. & Finn, O. J. Rapid, comprehensive analysis of human cytokine mRNA and its application to the study of acute renal allograft rejection. Hum. Immunol. 43, 113–128 (1995).
Strehlau, J. et al. Quantitative detection of immune activation transcripts as a diagnostic tool in kidney transplantation. Proc. Natl Acad. Sci. USA 94, 695–700 (1997).
Reeve, J. et al. Molecular diagnosis of T cell-mediated rejection in human kidney transplant biopsies. Am. J. Transplant. 13, 645–655 (2013).
Famulski, K. S. et al. Kidney transplants with progressing chronic diseases express high levels of acute kidney injury transcripts. Am. J. Transplant. 13, 634–644 (2013).
Sellares, J. et al. Molecular diagnosis of antibody-mediated rejection in human kidney transplants. Am. J. Transplant. 13, 971–983 (2013).
Li, L. et al. A peripheral blood diagnostic test for acute rejection in renal transplantation. Am. J. Transplant. 12, 2710–2718 (2012).
Keslar, K. S. et al. Multicenter evaluation of a standardized protocol for noninvasive gene expression profiling. Am. J. Transplant. 13, 1891–1897 (2013).
Hartono, C., Muthukumar, T. & Suthanthiran, M. Noninvasive diagnosis of acute rejection of renal allografts. Curr. Opin. Organ Transplant. 15, 35–41 (2010).
Li, B. et al. Noninvasive diagnosis of renal-allograft rejection by measurement of messenger RNA for perforin and granzyme B in urine. N. Engl. J. Med. 344, 947–954 (2001).
Galante, N. Z. et al. Noninvasive immune monitoring assessed by flow cytometry and real time RT-PCR in urine of renal transplantation recipients. Transpl. Immunol. 16, 73–80 (2006).
Muthukumar, T. et al. Serine proteinase inhibitor-9, an endogenous blocker of granzyme B/perforin lytic pathway, is hyperexpressed during acute rejection of renal allografts. Transplantation 75, 1565–1570 (2003).
Yannaraki, M. et al. Urinary cytotoxic molecular markers for a noninvasive diagnosis in acute renal transplant rejection. Transpl. Int. 19, 759–768 (2006).
Muthukumar, T. et al. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N. Engl. J. Med. 353, 2342–2351 (2005).
Afaneh, C. et al. Urinary cell levels of mRNA for OX40, OX40L, PD-1, PD-L1, or PD-L2 and acute rejection of human renal allografts. Transplantation 90, 1381–1387 (2010).
Suthanthiran, M. et al. Urinary-cell mRNA profile and acute cellular rejection in kidney allografts. N. Engl. J. Med. 369, 20–31 (2013).
Manfro, R. C. et al. Noninvasive TIM-3 messenger RNA evaluation in renal transplant recipients with graft dysfunction. Transplantation 86, 1869–1874 (2008).
Renesto, P. G., Ponciano, V. C., Cenedeze, M. A., Saraiva Camara, N. O. & Pacheco-Silva, A. High expression of TIM-3 mRNA in urinary cells from kidney transplant recipients with acute rejection. Am. J. Transplant. 7, 1661–1665 (2007).
Luo, Y., Shi, B., Qian, Y., Bai, H. & Chang, J. Sequential monitoring of TIM-3 gene expression in peripheral blood for diagnostic and prognostic evaluation of acute rejection in renal graft recipients. Transplant. Proc. 43, 3669–3674 (2011).
Lo, D. J. et al. Chemokines and their receptors in human renal allotransplantation. Transplantation 91, 70–77 (2011).
el-Sawy, T., Fahmy, N. M. & Fairchild, R. L. Chemokines: directing leukocyte infiltration into allografts. Curr. Opin. Immunol. 14, 562–568 (2002).
Hu, H. et al. Elevation of CXCR3-binding chemokines in urine indicates acute renal-allograft dysfunction. Am. J. Transplant. 4, 432–437 (2004).
Dufour, J. H. et al. IFN-γ-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J. Immunol. 168, 3195–3204 (2002).
Miura, M. et al. Monokine induced by IFN-γ is a dominant factor directing T cells into murine cardiac allografts during acute rejection. J. Immunol. 167, 3494–3504 (2001).
Hu, H., Kwun, J., Aizenstein, B. D. & Knechtle, S. J. Noninvasive detection of acute and chronic injuries in human renal transplant by elevation of multiple cytokines/chemokines in urine. Transplantation 87, 1814–1820 (2009).
Schaub, S. et al. Urinary CXCL9 and CXCL10 levels correlate with the extent of subclinical tubulitis. Am. J. Transplant. 9, 1347–1353 (2009).
Jackson, J. A. et al. Urinary chemokines CXCL9 and CXCL10 are noninvasive markers of renal allograft rejection and BK viral infection. Am. J. Transplant. 11, 2228–2234 (2011).
Hricik, D. E. et al. Multicenter validation of urinary CXCL9 as a risk-stratifying biomarker for kidney transplant injury. Am. J. Transplant. 13, 2634–2644 (2013).
Ho, J. et al. Early urinary CCL2 is associated with the later development of interstitial fibrosis and tubular atrophy in renal allografts. Transplantation 90, 394–400 (2010).
Ho, J. et al. Increased urinary CCL2:Cr ratio at 6 months is associated with late renal allograft loss. Transplantation 95, 595–602 (2013).
Guo, H., Ingolia, N. T., Weissman, J. S. & Bartel, D. P. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466, 835–840 (2010).
Sui, W. et al. Microarray analysis of microRNA expression in acute rejection after renal transplantation. Transpl. Immunol. 19, 81–85 (2008).
Anglicheau, D. et al. MicroRNA expression profiles predictive of human renal allograft status. Proc. Natl Acad. Sci. USA 106, 5330–5335 (2009).
Lorenzen, J. M. et al. Urinary miR-210 as a mediator of acute T-cell mediated rejection in renal allograft recipients. Am. J. Transplant. 11, 2221–2227 (2011).
Valujskikh, A. & Lakkis, F. G. In remembrance of things past: memory T cells and transplant rejection. Immunol. Rev. 196, 65–74 (2003).
Kirk, A. D., Ibrahim, M. A., Bollinger, R. R., Dawson, D. V. & Finn, O. J. Renal allograft-infiltrating lymphocytes. A prospective analysis of in vitro growth characteristics and clinical relevance. Transplantation 53, 329–338 (1992).
Heeger, P. S. et al. Pretransplant frequency of donor-specific, IFN-γ-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J. Immunol. 163, 2267–2275 (1999).
Kim, S. H. et al. Pretransplant donor-specific interferon-γ ELISPOT assay predicts acute rejection episodes in renal transplant recipients. Transplant. Proc. 39, 3057–3060 (2007).
Nather, B. J. et al. Modified ELISPOT technique—highly significant inverse correlation of post-Tx donor-reactive IFNγ-producing cell frequencies with 6 and 12 months graft function in kidney transplant recipients. Transpl. Immunol. 16, 232–237 (2006).
Nickel, P. et al. Enzyme-linked immunosorbent spot assay for donor-reactive interferon-γ-producing cells identifies T-cell presensitization and correlates with graft function at 6 and 12 months in renal-transplant recipients. Transplantation 78, 1640–1646 (2004).
Bestard, O. et al. Cross-validation of IFN-γ ELISPOT assay for measuring alloreactive memory/effector T cell responses in renal transplant recipients. Am. J. Transplant. 13, 1880–1890 (2013).
Kirk, A. D. et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (Campath-1H). Transplantation 76, 120–129 (2003).
Kowalski, R. J. et al. Assessing relative risks of infection and rejection: a meta-analysis using an immune function assay. Transplantation 82, 663–668 (2006).
Egli, A., Humar, A. & Kumar, D. State-of-the-art monitoring of cytomegalovirus-specific cell-mediated immunity after organ transplant: a primer for the clinician. Clin. Infect. Dis. 55, 1678–1689 (2012).
Ginevri, F. et al. Prospective monitoring of polyomavirus BK replication and impact of pre-emptive intervention in pediatric kidney recipients. Am. J. Transplant. 7, 2727–2735 (2007).
Ritta, M. et al. Evaluation of Epstein–Barr virus-specific immunologic response in solid organ transplant recipients with an enzyme-linked ImmunoSpot assay. Transplant. Proc. 45, 2754–2757 (2013).
Abate, D. et al. Comparison of cytomegalovirus (CMV) enzyme-linked immunosorbent spot and CMV QuantiFERON γ interferon-releasing assays in assessing risk of CMV infection in kidney transplant recipients. J. Clin. Microbiol. 51, 2501–2507 (2013).
Walker, S. et al. Ex vivo monitoring of human cytomegalovirus-specific CD8+ T-cell responses using QuantiFERON-CMV. Transpl. Infect. Dis. 9, 165–170 (2007).
Newell, K. A. et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J. Clin. Invest. 120, 1836–1847 (2010).
Sagoo, P. et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J. Clin. Invest. 120, 1848–1861 (2010).
Brouard, S. et al. Identification of a gene expression profile associated with operational tolerance among a selected group of stable kidney transplant patients. Transpl. Int. 24, 536–547 (2011).
Patel, R. & Terasaki, P. I. Significance of the positive crossmatch test in kidney transplantation. N. Engl. J. Med. 280, 735–739 (1969).
Gebel, H. M. & Bray, R. A. The evolution and clinical impact of human leukocyte antigen technology. Curr. Opin. Nephrol. Hypertens. 19, 598–602 (2010).
Terasaki, P. I., Ozawa, M. & Castro, R. Four-year follow-up of a prospective trial of HLA and MICA antibodies on kidney graft survival. Am. J. Transplant. 7, 408–415 (2007).
Wiebe, C. et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am. J. Transplant. 12, 1157–1167 (2012).
Reed, E. F. et al. Comprehensive assessment and standardization of solid phase multiplex-bead arrays for the detection of antibodies to, HLA. Am. J. Transplant. 13, 1859–1870 (2013).
Archdeacon, P. et al. Summary of FDA antibody-mediated rejection workshop. Am. J. Transplant. 11, 896–906 (2011).
Kirk, A. D. Meteorology and tolerance. Am. J. Transplant. 6, 645–646 (2006).
Morel, P. A., Ta'asan, S., Morel, B. F., Kirschner, D. E. & Flynn, J. L. New insights into mathematical modeling of the immune system. Immunol. Res. 36, 157–165 (2006).
Acknowledgements
A. D. Kirk is supported by NIH grant U01AI077821, FDA grant FD003539 and by the Georgia Research Alliance.
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article, provided substantial contributions to discussions of its content, wrote the article and undertook review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lo, D., Kaplan, B. & Kirk, A. Biomarkers for kidney transplant rejection. Nat Rev Nephrol 10, 215–225 (2014). https://doi.org/10.1038/nrneph.2013.281
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2013.281
This article is cited by
-
Therapeutic Potential of Skin Stem Cells and Cells of Skin Origin: Effects of Botanical Drugs Derived from Traditional Medicine
Stem Cell Reviews and Reports (2022)
-
Circulating endothelial cells transiently increase in peripheral blood after kidney transplantation
Scientific Reports (2021)
-
Urinary vitronectin identifies patients with high levels of fibrosis in kidney grafts
Journal of Nephrology (2021)
-
Biomarkers for PTLD diagnosis and therapies
Pediatric Nephrology (2020)
-
Endogenous intronic antisense long non-coding RNA, MGAT3-AS1, and kidney transplantation
Scientific Reports (2019)