Key Points
-
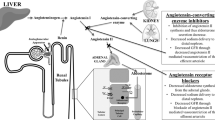
Renin–angiotensin–aldosterone system (RAAS) blockade using angiotensin-converting-enzyme inhibitors or angiotensin-receptor blockers, which have antihypertensive and antialbuminuric properties, has become the cornerstone of treatment of diabetic renal disease
-
As single-agent RAAS blockade does not completely block RAAS activation the residual renal risk of treated patients is high
-
Data from clinical trials of dual RAAS blockade (including the use of aldosterone blockers or renin inhibitors) are contradictory and safety remains a concern
-
Possible alternatives to dual RAAS blockade include restricting dietary sodium, increasing drug doses or using novel drugs to block the deleterious effects of RAAS activation whilst preserving its physiological role
-
Novel omic techniques might enable the identification of patients who are most likely to benefit from RAAS blockade and the individual tailoring of interventions
Abstract
The renin–angiotensin–aldosterone system (RAAS) has a key role in the regulation of blood pressure, sodium and water balance, and cardiovascular and renal homeostasis. In diabetic nephropathy, excessive activation of the RAAS results in progressive renal damage. RAAS blockade using angiotensin-converting-enzyme inhibitors or angiotensin-receptor blockers is the cornerstone of treatment of diabetic renal disease. Alternative RAAS-blockade strategies include renin inhibition and aldosterone blockade. Data from small initial studies of these agents are promising. However, single-agent interventions do not fully block the RAAS and patients treated with these therapies remain at high residual renal risk. Approaches to optimize drug responses include dietary changes and increasing dosages. The theoretically attractive option of combining different RAAS interventions has also been tested in clinical trials but long-term outcomes were disappointing. However, dual RAAS blockade might represent a good therapeutic option for specific patients. A better knowledge of the pathophysiology of the RAAS is crucial to fully understand the mechanisms of action of RAAS blockers and to exploit their renoprotective effects. Moreover, lifestyle interventions or diagnostic tools might be used to optimize RAAS blockade and identify those patients who are most likely to benefit from the therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
11 March 2014
In the February 2014 issue of Nature Reviews Nephrology, the key in Figure 4 of this article was labelled incorrectly. The correct labelling is as follows: pale green <1; dark green 1–2, blue 2–3; purple >3.5. This error has been corrected online.
References
Collins, A. J. et al. US Renal Data System 2012 Annual Data Report. Am. J. Kidney Dis. 61 (Suppl. 1), e1–e476 (2013).
Gross, J. L. et al. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 28, 164–176 (2005).
Grundy, S. M. et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 100, 1134–1146 (1999).
Lopes-Virella, M. F. et al. Risk factors related to inflammation and endothelial dysfunction in the DCCT/EDIC cohort and their relationship with nephropathy and macrovascular complications. Diabetes Care 31, 2006–2012 (2008).
Kanwar, Y. S. et al. Diabetic nephropathy: mechanisms of renal disease progression. Exp. Biol. Med. (Maywood) 233, 4–11 (2008).
Schrijvers, B. F., De Vriese, A. S. & Flyvbjerg, A. From hyperglycemia to diabetic kidney disease: the role of metabolic, hemodynamic, intracellular factors and growth factors/cytokines. Endocr. Rev. 25, 971–1010 (2004).
Atlas, S. A. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J. Manag. Care Pharm. 13, 9–20 (2007).
Ruggenenti, P., Cravedi, P. & Remuzzi, G. The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat. Rev. Nephrol. 6, 319–330 (2010).
Giacchetti, G., Sechi, L. A., Rilli, S. & Carey, R. M. The renin-angiotensin-aldosterone system, glucose metabolism and diabetes. Trends Endocrinol. Metab. 16, 120–126 (2005).
Kagami, S., Border, W. A., Miller, D. E. & Noble, N. A. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-β expression in rat glomerular mesangial cells. J. Clin. Invest. 93, 2431–2437 (1994).
Ruiz-Ortega, M. et al. Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol. Dial. Transplant. 21, 16–20 (2006).
Siragy, H. M. & Carey, R. M. Role of the intrarenal renin-angiotensin-aldosterone system in chronic kidney disease. Am. J. Nephrol. 31, 541–550 (2010).
Adler, A. I. et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 63, 225–232 (2003).
Knowler, W. C. et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 346, 393–403 (2002).
Tuck, M. L. et al. Insulin stimulates endogenous angiotensin II production via a mitogen-activated protein kinase pathway in vascular smooth muscle cells. J. Hypertens. 22, 1779–1785 (2004).
Hilgers, K. F. & Veelken, R. Type 2 diabetic nephropathy: never too early to treat? J. Am. Soc. Nephrol. 16, 574–575 (2005).
Yusuf, S. et al. Ramipril and the development of diabetes. JAMA 286, 1882–1885 (2001).
Abuissa, H., Jones, P. G., Marso, S. P. & O'Keefe, J. H. Jr. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes: a meta-analysis of randomized clinical trials. J. Am. Coll. Cardiol. 46, 821–826 (2005).
The DREAM Trial Investigators. Effect of ramipril on the incidence of diabetes. N. Engl. J. Med. 355, 1551–1562 (2006).
The NAVIGATOR Study Group. Effect of valsartan on the incidence of diabetes and cardiovascular events. N. Engl. J. Med. 362, 1477–1490 (2010).
Ravid, M. et al. Use of enalapril to attenuate decline in renal function in normotensive, normoalbuminuric patients with type 2 diabetes mellitus. A randomized, controlled trial. Ann. Intern. Med. 128, 982–988 (1998).
Ruggenenti, P. et al. Preventing microalbuminuria in type 2 diabetes. N. Engl. J. Med. 351, 1941–1951 (2004).
Haller, H. et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N. Engl. J. Med. 364, 907–917 (2011).
Parving, H. H. et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N. Engl. J. Med. 345, 870–878 (2001).
Makino, H. et al. Prevention of transition from incipient to overt nephropathy with telmisartan in patients with type 2 diabetes. Diabetes Care 30, 1577–1578 (2007).
Patel, A. et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 370, 829–840 (2007).
Lewis, E. J., Hunsicker, L. G., Bain, R. P. & Rohde, R. D. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N. Engl. J. Med. 329, 1456–1462 (1993).
Lewis, E. J. et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 345, 851–860 (2001).
Brenner, B. M. et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 345, 861–869 (2001).
de Zeeuw, D. et al. Renal risk and renoprotection among ethnic groups with type 2 diabetic nephropathy: a post hoc analysis of RENAAL. Kidney Int. 69, 1675–1682 (2006).
Imai, E. et al. Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy: a multicentre, randomised, placebo-controlled study. Diabetologia 54, 2978–2986 (2011).
Mauer, M. et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N. Engl. J. Med. 361, 40–51 (2009).
Barnett, A. H. et al. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N. Engl. J. Med. 351, 1952–1961 (2004).
Lacourciere, Y. et al. Long-term comparison of losartan and enalapril on kidney function in hypertensive type 2 diabetics with early nephropathy. Kidney Int. 58, 762–769 (2000).
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 3, 1–150 (2013).
Parving, H. H., Andersen, A. R., Smidt, U. M. & Svendsen, P. A. Early aggressive antihypertensive treatment reduces rate of decline in kidney function in diabetic nephropathy. Lancet 1, 1175–1179 (1983).
Bakris, G. L. et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. Am. J. Kidney Dis. 36, 646–661 (2000).
Bjorck, S., Mulec, H., Johnsen, S. A., Norden, G. & Aurell, M. Renal protective effect of enalapril in diabetic nephropathy. BMJ 304, 339–343 (1992).
[No authors listed] Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 317, 713–720 (1998).
Nielsen, F. S. et al. Long-term effect of lisinopril and atenolol on kidney function in hypertensive NIDDM subjects with diabetic nephropathy. Diabetes 46, 1182–1188 (1997).
Holmer, S. R. et al. Marked suppression of renin levels by β-receptor blocker in patients treated with standard heart failure therapy: a potential mechanism of benefit from β-blockade. J. Intern. Med. 249, 167–172 (2001).
Atkins, R. C. et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am. J. Kidney Dis. 45, 281–287 (2005).
de Zeeuw, D. et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int. 65, 2309–2320 (2004).
Hellemons, M. E. et al. Initial angiotensin receptor blockade-induced decrease in albuminuria is associated with long-term renal outcome in type 2 diabetic patients with microalbuminuria: a post hoc analysis of the IRMA-2 trial. Diabetes Care 34, 2078–2083 (2011).
Perkins, B. A., Ficociello, L. H., Roshan, B., Warram, J. H. & Krolewski, A. S. In patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuria. Kidney Int. 77, 57–64 (2010).
Apperloo, A. J., de Zeeuw, D. & de Jong, P. E. A short-term antihypertensive treatment-induced fall in glomerular filtration rate predicts long-term stability of renal function. Kidney Int. 51, 793–797 (1997).
Holtkamp, F. A. et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 80, 282–287 (2011).
Hansen, H. P. et al. Increased glomerular filtration rate after withdrawal of long-term antihypertensive treatment in diabetic nephropathy. Kidney Int. 47, 1726–1731 (1995).
Eijkelkamp, W. B. et al. Albuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy: post hoc analysis from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. J. Am. Soc. Nephrol. 18, 1540–1546 (2007).
Rossing, K., Schjoedt, K. J., Jensen, B. R., Boomsma, F. & Parving, H. H. Enhanced renoprotective effects of ultrahigh doses of irbesartan in patients with type 2 diabetes and microalbuminuria. Kidney Int. 68, 1190–1198 (2005).
Hou, F. F. et al. Renoprotection of Optimal Antiproteinuric Doses (ROAD) Study: a randomized controlled study of benazepril and losartan in chronic renal insufficiency. J. Am. Soc. Nephrol. 18, 1889–1898 (2007).
Forclaz, A., Maillard, M., Nussberger, J., Brunner, H. R. & Burnier, M. Angiotensin II receptor blockade: is there truly a benefit of adding an ACE inhibitor? Hypertension 41, 31–36 (2003).
Kunz, R., Friedrich, C., Wolbers, M. & Mann, J. F. Meta-analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann. Intern. Med. 148, 30–48 (2008).
Mann, J. F. et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 372, 547–553 (2008).
Bomback, A. S. & Klemmer, P. J. The incidence and implications of aldosterone breakthrough. Nat. Clin. Pract. Nephrol. 3, 486–492 (2007).
Epstein, M. Aldosterone as a mediator of progressive renal disease: pathogenetic and clinical implications. Am. J. Kidney Dis. 37, 677–688 (2001).
Gilbert, K. C. & Brown, N. J. Aldosterone and inflammation. Curr. Opin. Endocrinol. Diabetes Obes. 17, 199–204 (2010).
Waanders, F. et al. Aldosterone, from (patho)physiology to treatment in cardiovascular and renal damage. Curr. Vasc. Pharmacol. 9, 594–605 (2011).
Schjoedt, K. J., Andersen, S., Rossing, P., Tarnow, L. & Parving, H. H. Aldosterone escape during blockade of the renin–angiotensin–aldosterone system in diabetic nephropathy is associated with enhanced decline in glomerular filtration rate. Diabetologia 47, 1936–1939 (2004).
Bianchi, S., Bigazzi, R. & Campese, V. M. Antagonists of aldosterone and proteinuria in patients with CKD: an uncontrolled pilot study. Am. J. Kidney Dis. 46, 45–51 (2005).
Epstein, M. et al. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin. J. Am. Soc. Nephrol. 1, 940–951 (2006).
Roscioni, S. S., de Zeeuw, D., Bakker, S. J. & Lambers Heerspink, H. J. Management of hyperkalaemia consequent to mineralocorticoid-receptor antagonist therapy. Nat. Rev. Nephrol. 8, 691–699 (2012).
van den Meiracker, A. H. et al. Spironolactone in type 2 diabetic nephropathy: effects on proteinuria, blood pressure and renal function. J. Hypertens. 24, 2285–2292 (2006).
Morales, E. et al. Renoprotective effects of mineralocorticoid receptor blockers in patients with proteinuric kidney diseases. Nephrol. Dial. Transplant. 28, 405–412 (2013).
Mehdi, U. F., Adams-Huet, B., Raskin, P., Vega, G. L. & Toto, R. D. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J. Am. Soc. Nephrol. 20, 2641–2650 (2009).
Pitt, B. et al. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur. Heart J. 32, 820–828 (2011).
Pitt, B. et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94–8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur. Heart J. 34, 2453–2463 (2013).
Nariai, T. et al. SM-368229, a novel selective and potent non-steroidal mineralocorticoid receptor antagonist with strong urinary Na+ excretion activity. J. Pharmacol. Sci. 115, 346–353 (2011).
Nariai, T. et al. SM-368229, a novel promising mineralocorticoid receptor antagonist, shows antihypertensive efficacy with minimal effect on serum potassium level in rats. J. Cardiovasc. Pharmacol. 59, 458–464 (2012).
Bassett, M. H., White, P. C. & Rainey, W. E. The regulation of aldosterone synthase expression. Mol. Cell Endocrinol. 217, 67–74 (2004).
Amar, L. et al. Aldosterone synthase inhibition with LCI699: a proof-of-concept study in patients with primary aldosteronism. Hypertension 56, 831–838 (2010).
Lea, W. B. et al. Aldosterone antagonism or synthase inhibition reduces end-organ damage induced by treatment with angiotensin and high salt. Kidney Int. 75, 936–944 (2009).
Calhoun, D. A. et al. Effects of a novel aldosterone synthase inhibitor for treatment of primary hypertension: results of a randomized, double-blind, placebo- and active-controlled phase 2 trial. Circulation 124, 1945–1955 (2011).
Nguyen, G. & Muller, D. N. The biology of the (pro)renin receptor. J. Am. Soc. Nephrol. 21, 18–23 (2010).
van Paassen, P., de Zeeuw, D., Navis, G. & de Jong, P. E. Renal and systemic effects of continued treatment with renin inhibitor remikiren in hypertensive patients with normal and impaired renal function. Nephrol. Dial. Transplant. 15, 637–643 (2000).
Kelly, D. J., Zhang, Y., Moe, G., Naik, G. & Gilbert, R. E. Aliskiren, a novel renin inhibitor, is renoprotective in a model of advanced diabetic nephropathy in rats. Diabetologia 50, 2398–2404 (2007).
Persson, F. et al. Time course of the antiproteinuric and antihypertensive effects of direct renin inhibition in type 2 diabetes. Kidney Int. 73, 1419–1425 (2008).
Persson, F. et al. Aliskiren in combination with losartan reduces albuminuria independent of baseline blood pressure in patients with type 2 diabetes and nephropathy. Clin. J. Am. Soc. Nephrol. 6, 1025–1031 (2011).
Persson, F. et al. Renal effects of aliskiren compared with and in combination with irbesartan in patients with type 2 diabetes, hypertension, and albuminuria. Diabetes Care 32, 1873–1879 (2009).
Parving, H. H. et al. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N. Engl. J. Med. 358, 2433–2446 (2008).
Parving, H. H. et al. Dual renin-angiotensin system blockade and kidney disease. J. Am. Coll. Cardiol. 54, 278–279 (2009).
Ruggenenti, P. & Remuzzi, G. Proteinuria: is the ONTARGET renal substudy actually off target? Nat. Rev. Nephrol. 5, 436–437 (2009).
Parving, H. H. et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N. Engl. J. Med. 367, 2204–2213 (2012).
Fried, L. F. et al. Design of combination angiotensin receptor blocker and angiotensin-converting enzyme inhibitor for treatment of diabetic nephropathy (VA NEPHRON-D). Clin. J. Am. Soc. Nephrol. 4, 361–368 (2009).
Imai, E. et al. Effects of dual blockade of the renin-angiotensin system on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy and hypertension in the ORIENT: a post-hoc analysis (ORIENT-Hypertension). Hypertens. Res. http://dx.doi.org/10.1038/hr.2013.86.
Ruggenenti, P. & Remuzzi, G. Dual RAS blockade—controversy resolved. Nat. Rev. Nephrol. 9, 640 (2013).
Miao, Y. et al. Increased serum potassium affects renal outcomes: a post hoc analysis of the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. Diabetologia 54, 44–50 (2011).
Mohanram, A., Zhang, Z., Shahinfar, S., Lyle, P. A. & Toto, R. D. The effect of losartan on hemoglobin concentration and renal outcome in diabetic nephropathy of type 2 diabetes. Kidney Int. 73, 630–636 (2008).
Slagman, M. C. et al. Erythropoietin is reduced by combination of diuretic therapy and RAAS blockade in proteinuric renal patients with preserved renal function. Nephrol. Dial. Transplant. 25, 3256–3260 (2010).
Miao, Y. et al. Effect of a reduction in uric acid on renal outcomes during losartan treatment: a post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial. Hypertension 58, 2–7 (2011).
Di Raimondo, D. et al. Effects of ACE-inhibitors and angiotensin receptor blockers on inflammation. Curr. Pharm. Des. 18, 4385–4413 (2012).
Onuigbo, M. A. & Onuigbo, N. T. Worsening renal failure in older chronic kidney disease patients with renal artery stenosis concurrently on renin angiotensin aldosterone system blockade: a prospective 50-month Mayo-Health-System clinic analysis. QJM 101, 519–527 (2008).
Onuigbo, M. A. Radiographic contrast-induced nephropathy and patient mortality. Mayo Clin. Proc. 83, 1412–1413 (2008).
Onuigbo, M. A. Syndrome of rapid-onset end-stage renal disease: a new unrecognized pattern of CKD progression to ESRD. Ren. Fail. 32, 954–958 (2010).
Onuigbo, M. A. Evidence of the syndrome of rapid onset end-stage renal disease (SORO-ESRD) in the acute kidney injury (AKI) literature—preventable causes of AKI and SORO-ESRD—a call for re-engineering of nephrology practice paradigms. Ren. Fail. 35, 796–800 (2013).
Richer-Giudicelli, C. et al. Haemodynamic effects of dual blockade of the renin-angiotensin system in spontaneously hypertensive rats: influence of salt. J. Hypertens. 22, 619–627 (2004).
Lambers Heerspink, H. J. et al. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int. 82, 330–337 (2012).
Mark, A. L. The Bezold-Jarisch reflex revisited: clinical implications of inhibitory reflexes originating in the heart. J. Am. Coll. Cardiol. 1, 90–102 (1983).
Sever, P. Hypotension and ischaemic stroke associated with aliskiren in the ALTITUDE trial: sensitisation of the Bezold-Jarisch reflex? J. Renin Angiotensin Aldosterone Syst. 14, 1–2 (2013).
Lambers Heerspink, H. J., de Borst, M. H., Bakker, S. J. & Navis, G. J. Improving the efficacy of RAAS blockade in patients with chronic kidney disease. Nat. Rev. Nephrol. 9, 112–121 (2013).
Slagman, M. C. et al. Moderate dietary sodium restriction added to angiotensin converting enzyme inhibition compared with dual blockade in lowering proteinuria and blood pressure: randomised controlled trial. BMJ 343, d4366 (2011).
Vogt, L., Waanders, F., Boomsma, F., de Zeeuw, D. & Navis, G. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J. Am. Soc. Nephrol. 19, 999–1007 (2008).
Bader, M. ACE2, angiotensin-(1–7), and Mas: the other side of the coin. Pflugers Arch. 465, 79–85 (2013).
Ye, M. et al. Glomerular localization and expression of angiotensin-converting enzyme 2 and angiotensin-converting enzyme: implications for albuminuria in diabetes. J. Am. Soc. Nephrol. 17, 3067–3075 (2006).
Su, Z., Zimpelmann, J. & Burns, K. D. Angiotensin-(1–7) inhibits angiotensin II-stimulated phosphorylation of MAP kinases in proximal tubular cells. Kidney Int. 69, 2212–2218 (2006).
de Gasparo, M., Catt, K. J., Inagami, T., Wright, J. W. & Unger, T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol. Rev. 52, 415–472 (2000).
PRIORITY Consortium. EU Priority [online] (2013).
Author information
Authors and Affiliations
Contributions
S. S. Roscioni and H. J. Lambers Heerspink contributed equally to researching the data for the article and writing the manuscript. D. de Zeeuw reviewed and/or edited the manuscript before submission. All authors made a substantial contribution to discussions of the content.
Corresponding author
Ethics declarations
Competing interests
S. S. Roscioni declares no competing interests. H. J. Lambers Heerspink has consulted for AbbVie, Astellas, Johnson & Johnson, Reata Pharmaceuticals, and Vitae. D. de Zeeuw has consulted for AbbVie, Astellas, Chemocentryx, Johnson & Johnson, Novartis, Reata Pharmaceutical, Takeda, and VITAE.
Rights and permissions
About this article
Cite this article
Roscioni, S., Heerspink, H. & de Zeeuw, D. The effect of RAAS blockade on the progression of diabetic nephropathy. Nat Rev Nephrol 10, 77–87 (2014). https://doi.org/10.1038/nrneph.2013.251
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2013.251
This article is cited by
-
Alamandine/MrgD axis prevents TGF-β1-mediated fibroblast activation via regulation of aerobic glycolysis and mitophagy
Journal of Translational Medicine (2023)
-
In vivo mapping of hemodynamic responses mediated by tubuloglomerular feedback in hypertensive kidneys
Scientific Reports (2023)
-
Angiotensin II Receptor Antagonist, Valsartan, Has Beneficial Effect in Lung Metastasis of Colorectal Cancer Treated with Fluorouracil
Journal of Gastrointestinal Cancer (2023)
-
Traditional Chinese Medicine Fufang-Zhenzhu-Tiaozhi capsule prevents renal injury in diabetic minipigs with coronary heart disease
Chinese Medicine (2022)
-
Signaling pathways of chronic kidney diseases, implications for therapeutics
Signal Transduction and Targeted Therapy (2022)