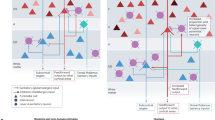
Key Points
-
Much more is now known about human cortical development than was known in 1970, when the Boulder Committee attempted to standardize the heterogeneous and confusing nomenclature that was used in the field. In light of this, a revised standardization is needed.
-
A transient layer with a diverse population of neurons forms between the neuroepithelium and the pial surface of the dorsal telencephalon before the appearance of the cortical plate (CP). The term preplate, which is already widely used, should be adopted for this layer.
-
The subventricular zone appears as a distinctive proliferative layer before the emergence of the CP, earlier than previously recognized.
-
There is no distinct cell-sparse layer under the pial surface before the CP forms. Thus, the term marginal zone should be used only after the appearance of the CP, to refer to the residual superficial part of the preplate, which becomes the layer 1 of the mature cortex.
-
The term intermediate zone (IZ) has been used in various ways. In future it should be reserved for the heterogeneous compartment that lies between the proliferative layers and the postmigratory cells above. The IZ contains radially and tangentially migrating cells and a thickening layer of extrinsic axons that eventually constitutes the white matter.
-
The subplate (SP) is a distinct and functionally important transient layer, located directly below the cortical plate, which was not recognized by the Boulder Committee. In rodents and carnivores most SP neurons are born before the first CP cells. In humans, preplate cells also contribute to the SP, but its substantial thickening at later stages probably involves the addition of later-born neurons.
Abstract
In 1970 the Boulder Committee described the basic principles of the development of the CNS, derived from observations on the human embryonic cerebrum. Since then, numerous studies have significantly advanced our knowledge of the timing, sequence and complexity of developmental events, and revealed important inter-species differences. We review current data on the development of the human cerebral cortex and update the classical model of how the structure that makes us human is formed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Brodmann, K. Beitraege zur histologischen lokalisation der grosshirnrinde. VI. Mitteilung: die cortexgliederung des menschen. J. Psychol. Neurol. (Lzp.), 10, 231–246 (1908).
Sidman, R. L. & Rakic, P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 62, 1–35 (1973). This was the first review of the modes and patterns of neuronal migration in the embryonic and fetal human brain, and it remains the most comprehensive. It places a special emphasis on the cerebral and cerebellar cortices.
Marin, O. & Rubenstein, J. L. Cell migration in the forebrain. Annu. Rev. Neurosci. 26, 441–483 (2003). This is a comprehensive and highly informative review on the pattern of neuronal migration to the cerebral cortex that places a particular emphasis on the tangential migration of GABAergic interneurons from the ganglionic eminence of the ventral telencephalon (see also reference 154).
The Boulder Committee. Embryonic vertebrate central nervous system: revised terminology. Anat. Rec 166, 257–261 (1970). This landmark article recommended a clarified terminology for the transient embryonic zones, as presented in P.R.'s model based on human Golgi-stained embryonic cerebral tissue.
Haydar, T. F., Kuan, C. Y., Flavell, R. A. & Rakic, P. The role of cell death in regulating the size and shape of the mammalian forebrain. Cereb. Cortex 9, 621–626 (1999).
Shen, Q. et al. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nature Neurosci. 9, 743–751 (2006). This is a recent review of the multiple lines of evidence that indicate that the basic neuronal phenotypes are determined at the time of the last cell division in the proliferative zones (see also references 31 and 137).
Hatten, M. E. & Mason, C. A. Mechanisms of glial-guided neuronal migration in vitro and in vivo. Experientia 46, 907–916 (1990).
Nadarajah, B. & Parnavelas, J. G. Modes of neuronal migration in the developing cerebral cortex. Nature Rev. Neurosci. 3, 423–432 (2002).
Gleeson, J. G. & Walsh, C. A. Neuronal migration disorders: from genetic diseases to developmental mechanisms. Trends Neurosci. 23, 352–359 (2000).
Molyneaux, B. J., Arlotta, P., Menezes, J. R. & Macklis, J. D. Neuronal subtype specification in the cerebral cortex. Nature Rev. Neurosci. 8, 427–437 (2007).
Rasin, M. R. et al. Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nature Neurosci. 10, 819–827 (2007).
Guillemot, F. Spatial and temporal specification of neural fates by transcription factor codes. Development 134, 3771–3780 (2007).
Bystron, I., Rakic, P., Molnar, Z. & Blakemore, C. The first neurons of the human cerebral cortex. Nature Neurosci. 9, 880–886 (2006). This study made use of advanced immunocytochemical methods to study fresh tissues from early-stage embryonic human telencephalon, and revealed previously unrecognized 'predecessor neurons'. The evolutionary and medical significance of the findings are discussed.
Carney, R. S., Bystron, I., Lopez-Bendito, G. & Molnár, Z. Comparative analysis of extra-ventricular mitoses at early stages of cortical development in rat and human. Brain Struct. Funct. 212, 37–54 (2007).
Smart, I. H., Dehay, C., Giroud, P., Berland, M. & Kennedy, H. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb. Cortex 12, 37–53 (2002).
Zecevic, N., Chen, Y. & Filipovic, R. Contributions of cortical subventricular zone to the development of the human cerebral cortex. J. Comp. Neurol. 491, 109–122 (2005).
Bielle, F. et al. Multiple origins of Cajal-Retzius cells at the borders of the developing pallium. Nature Neurosci. 8, 1002–1012 (2005).
O'Rourke, N. A., Chenn, A. & McConnell, S. K. Postmitotic neurons migrate tangentially in the cortical ventricular zone. Development 124, 997–1005 (1997).
Lopez-Bendito, G. & Molnar, Z. Thalamocortical development: how are we going to get there? Nature Rev. Neurosci. 4, 276–289 (2003).
Polleux, F. Genetic mechanisms specifying cortical connectivity: let's make some projections together. Neuron 46, 395–400 (2005).
Molnar, Z. & Blakemore, C. How do thalamic axons find their way to the cortex? Trends Neurosci. 18, 389–397 (1995).
Kriegstein, A. & Parnavelas, J. G. Progress in corticogenesis. Cereb. Cortex 16 (Suppl. 1), i1–i2 (2006).
Rubenstein, J. L. & Rakic, P. Genetic control of cortical development. Cereb. Cortex 9, 521–523 (1999).
Larsen, W. J. Human Embryology (Churchill Livingstone, 2001).
Jacobson, M. Developmental Neurobiology (Plenum, New York, 1991).
Sheperd, G. M. Neurobiology (Oxford Univ. Press, Oxford, 1994).
Allendoerfer, K. L. & Shatz, C. J. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu. Rev. Neurosci. 17, 185–218 (1994).
O'Leary, D. D. & Nakagawa, Y. Patterning centers, regulatory genes and extrinsic mechanisms controlling arealization of the neocortex. Curr. Opin. Neurobiol. 12, 14–25 (2002). This article, together with references 6 and 136, provided evidence that the initial neuronal phenotypes for the prospective species-specific pattern and size of cytoarchitectonic areas are indicated early in the proliferative zones, as outlined in the protomap hypothesis in reference 31.
Dehay, C. & Kennedy, H. Cell-cycle control and cortical development. Nature Rev. Neurosci. 8, 438–450 (2007).
O'Rahilly, M. The Embryonic Human Brain: An Atlas of Developmental Stages (Wiley-Liss, New York, 1999).
Rakic, P. Specification of cerebral cortical areas. Science 241, 170–176 (1988). This paper reviews the initial evidence that the phenotype of cortical neurons, as well as their laminar and areal position, is specified at the time of their last division in the proliferative zones and only later influenced by incoming axonal input (the protomap hypthesis). It also proposed the radial unit hypothesis of cortical development and evolution that has recently been supported by genetic and cell biological methods (see references 28, 136 and 138).
Sauer, F. C. Mitosis in the neural tube. J. Comp. Neurol. 62, 377–405 (1935).
Caviness, V. S. Jr & Rakic, P. Mechanisms of cortical development: a view from mutations in mice. Annu. Rev. Neurosci. 1, 297–326 (1978). This paper reviews the early evidence that the basic neuronal phenotype reflects the time of neuron origin, irrespective of the neurons' subsequent laminar positions. This finding, together with those presented in reference 136, led to the conclusion that neurons attract appropriate thalamic input, contrary to the previous assumption that they are initially equipotent and specified by the type of input.
Gotz, M. & Huttner, W. B. The cell biology of neurogenesis. Nature Rev. Mol. Cell Biol. 6, 777–788 (2005).
Gotz, M. & Barde, Y. A. Radial glial cells defined and major intermediates between embryonic stem cells and CNS neurons. Neuron 46, 369–372 (2005).
Iacopetti, P. et al. Expression of the antiproliferative gene TIS21 at the onset of neurogenesis identifies single neuroepithelial cells that switch from proliferative to neuron-generating division. Proc. Natl Acad. Sci. USA 96, 4639–4644 (1999).
Rakic, P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 18, 383–388 (1995).
Chenn, A. & McConnell, S. K. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell 82, 631–641 (1995).
Garcia-Moreno, F., Lopez-Mascaraque, L. & De Carlos, J. A. Origins and migratory routes of murine Cajal-Retzius cells. J. Comp. Neurol. 500, 419–432 (2007).
Noctor, S. C., Flint, A. C., Weissman, T. A., Dammerman, R. S. & Kriegstein, A. R. Neurons derived from radial glial cells establish radial units in neocortex. Nature 409, 714–720 (2001). This study provided experimental evidence, obtained from live images of retrovirally labelled cells in slices of embryonic mouse cerebral wall, that radial glial cells can produce neurons that directly (or after additional mitotic divisions) migrate and form radial columns in the overlying cortical plate.
Takahashi, T., Nowakowski, R. S. & Caviness, V. S. Jr. Early ontogeny of the secondary proliferative population of the embryonic murine cerebral wall. J. Neurosci. 15, 6058–6068 (1995). This is one of several articles by this group (see also reference 104) that provide a useful model of the kinetics of neuronal proliferation and allocation in the embryonic cerebral wall, which have implications in cortical expansion during evolution.
Cappello, S. et al. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nature Neurosci. 9, 1099–1107 (2006).
Hartfuss, E., Galli, R., Heins, N. & Gotz, M. Characterization of CNS precursor subtypes and radial glia. Dev. Biol. 229, 15–30 (2001).
Gotz, M., Stoykova, A. & Gruss, P. Pax6 controls radial glia differentiation in the cerebral cortex. Neuron 21, 1031–1044 (1998).
Zecevic, N. Specific characteristic of radial glia in the human fetal telencephalon. Glia 48, 27–35 (2004).
Howard, B., Chen, Y. & Zecevic, N. Cortical progenitor cells in the developing human telencephalon. Glia 53, 57–66 (2006).
Gal, J. S. et al. Molecular and morphological heterogeneity of neural precursors in the mouse neocortical proliferative zones. J. Neurosci. 26, 1045–1056 (2006).
Levitt, P., Cooper, M. L. & Rakic, P. Coexistence of neuronal and glial precursor cells in the cerebral ventricular zone of the fetal monkey: an ultrastructural immunoperoxidase analysis. J. Neurosci. 1, 27–39 (1981). This study provided the first evidence that neuronal and glial cell lines can be distinguished at the initial stages of corticoneurogenesis in primates, a finding that has been confirmed in human post-mortem material in references 45, 46 and 132.
Ostenfeld, T. & Svendsen, C. N. Requirement for neurogenesis to proceed through the division of neuronal progenitors following differentiation of epidermal growth factor and fibroblast growth factor-2-responsive human neural stem cells. Stem Cells 22, 798–811 (2004).
Mo, Z. et al. Human cortical neurons originate from radial glia and neuron-restricted progenitors. J. Neurosci. 27, 4132–4145 (2007).
Smart, I. H. Proliferative characteristics of the ependymal layer during the early development of the mouse neocortex: a pilot study based on recording the number, location and plane of cleavage of mitotic figures. J. Anat. 116, 67–91 (1973).
Haubensak, W., Attardo, A., Denk, W. & Huttner, W. B. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc. Natl Acad. Sci. USA 101, 3196–3201 (2004).
Miyata, T. et al. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development 131, 3133–3145 (2004).
Altman, J. & Bayer, S. A. Vertical compartmentation and cellular transformations in the germinal matrices of the embryonic rat cerebral cortex. Exp. Neurol. 107, 23–35 (1990).
Brazel, C. Y., Romanko, M. J., Rothstein, R. P. & Levison, S. W. Roles of the mammalian subventricular zone in brain development. Prog. Neurobiol. 69, 49–69 (2003).
Letinic, K., Zoncu, R. & Rakic, P. Origin of GABAergic neurons in the human neocortex. Nature 417, 645–649 (2002). This study used retroviral labelling in the supravital slices of the embryonic human forebrain to show that a large proportion of interneurons in this primate are generated in the local SVZ and migrate radially to the suprajacent cortex. The evolutionary and medical implications for human-specific psychiatric disorders are discussed.
Rakic, P. Developmental and evolutionary adaptations of cortical radial glia. Cereb. Cortex 13, 541–549 (2003).
Noctor, S. C., Martinez-Cerdeno, V., Ivic, L. & Kriegstein, A. R. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nature Neurosci. 7, 136–144 (2004).
Englund, C. et al. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J. Neurosci. 25, 247–251 (2005).
Mizutani, K., Yoon, K., Dang, L., Tokunaga, A. & Gaiano, N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature 449, 351–355 (2007).
Carpenter, M. K. et al. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp. Neurol. 172, 383–397 (2001).
Rakic, P. & Sidman, R. L. Supravital DNA synthesis in the developing human and mouse brain. J. Neuropathol. Exp. Neurol. 27, 246–276 (1968). This study, which made use of supravitally labelled DNA, provided the first experimental evidence that neurons in the embryonic human cerebral cortex are not born locally, but rather all arrive by active and selective migration from the increasingly distant proliferative zones.
Chan, W. Y., et al. Normal and abnormal development of the human cerebral cortex. Neuroembryol. 1, 78–90 (2002).
Bystron, I., Molnar, Z., Otellin, V. & Blakemore, C. Tangential networks of precocious neurons and early axonal outgrowth in the embryonic human forebrain. J. Neurosci. 25, 2781–2792 (2005).
Kriegstein, A., Noctor, S. & Martinez-Cerdeno, V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nature Rev. Neurosci. 7, 883–890 (2006).
Bayatti, N. et al. A molecular neuroanatomical study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cereb. Cortex 26 Oct 2007 (doi:10.1093/cercor/bhm184).
Quinn, J. C. et al. Pax6 controls cerebral cortical cell number by regulating exit from the cell cycle and specifies cortical cell identity by a cell autonomous mechanism. Dev. Biol. 302, 50–65 (2007).
Simonati, A., Tosati, C., Rosso, T., Piazzola, E. & Rizzuto, N. Cell proliferation and death: morphological evidence during corticogenesis in the developing human brain. Microsc. Res. Tech. 45, 341–352 (1999).
Valverde, F., De Carlos, J. A. & Lopez-Mascaraque, L. Time of origin and early fate of preplate cells in the cerebral cortex of the rat. Cereb. Cortex 5, 483–493 (1995).
Samuelsen, G. B. et al. The changing number of cells in the human fetal forebrain and its subdivisions: a stereological analysis. Cereb. Cortex 13, 115–122 (2003).
Larroche, J. C. The marginal layer in the neocortex of a 7 week-old human embryo. A light and electron microscopic study. Anat. Embryol. (Berl.) 162, 301–312 (1981).
Meyer, G., Schaaps, J. P., Moreau, L. & Goffinet, A. M. Embryonic and early fetal development of the human neocortex. J. Neurosci. 20, 1858–1868 (2000).
Stewart, G. R. & Pearlman, A. L. Fibronectin-like immunoreactivity in the developing cerebral cortex. J. Neurosci. 7, 3325–3333 (1987).
Super, H., Soriano, E. & Uylings, H. B. The functions of the preplate in development and evolution of the neocortex and hippocampus. Brain Res. Brain Res. Rev. 27, 40–64 (1998).
Marin-Padilla, M. Structural organization of the human cerebral cortex prior to the appearance of the cortical plate. Anat. Embryol. (Berl.) 168, 21–40 (1983).
Rickmann, M. & Wolff, J. R. Differentiation of 'preplate' neurons in the pallium of the rat. Bibl. Anat., 142–146 (1981).
Rakic, P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science 183, 425–427 (1974).
Angevine, J. B. Jr & Sidman, R. L. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature 192, 766–768 (1961).
Marin-Padilla, M. Cajal–Retzius cells and the development of the neocortex. Trends Neurosci. 21, 64–71 (1998).
Marin-Padilla, M. Dual origin of the mammalian neocortex and evolution of the cortical plate. Anat. Embryol. (Berl.) 152, 109–126 (1978).
Meyer, G., Goffinet, A. M. & Fairen, A. What is a Cajal–Retzius cell? A reassessment of a classical cell type based on recent observations in the developing neocortex. Cereb. Cortex 9, 765–775 (1999).
Hevner, R. F. & Zecevic, N. Pioneer Neurons and Interneurons in the Developing Subplate: Molecular Markers, Cell Birthdays, and Neurotransmitters (eds Erzurumlu, R., Guido, W. & Molnár, Z.) (Springer US, 2006).
Poliakov, G. I. in Development of the Cerebral Neocortex During the First Half of Intrauterine Life (ed. Sarkisov, S. A.) 22–52 (Medicina, Leningrad, 1965). This book was the most comprehensive histological study of the stages of cortical development in the embryonic and fetal human cerebrum. Excerpts from the book and some illustrations are available in reference 85.
Bayer, S. A. & Altman, J. Neocortical Development (Raven, New York, 1991).
Sidman, R. L. & Rakic, P. in Histology and Histopathology of the Nervous System (eds Haymaker, W. & Adams, R. D.) 3–145 (Springfield, Illinois, 1982).
Rakic, P., Cameron, R. S. & Komuro, H. Recognition, adhesion, transmembrane signaling and cell motility in guided neuronal migration. Curr. Opin. Neurobiol. 4, 63–69 (1994).
Gressens, P. Mechanisms and disturbances of neuronal migration. Pediatr. Res. 48, 725–730 (2000).
Frantz, G. D. & McConnell, S. K. Restriction of late cerebral cortical progenitors to an upper-layer fate. Neuron 17, 55–61 (1996).
Tarabykin, V., Stoykova, A., Usman, N. & Gruss, P. Cortical upper layer neurons derive from the subventricular zone as indicated by Svet1 gene expression. Development 128, 1983–1993 (2001).
Nieto, M. et al. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II-IV of the cerebral cortex. J. Comp. Neurol. 479, 168–180 (2004).
Rakic, S. & Zecevic, N. Emerging complexity of layer I in human cerebral cortex. Cereb. Cortex 13, 1072–1083 (2003).
Hill, R. S. & Walsh, C. A. Molecular insights into human brain evolution. Nature 437, 64–67 (2005).
Majdan, M. & Shatz, C. J. Effects of visual experience on activity-dependent gene regulation in cortex. Nature Neurosci. 9, 650–659 (2006).
Sengpiel, F. & Kind, P. C. The role of activity in development of the visual system. Curr. Biol. 12, R818–R826 (2002).
Kind, P. C. et al. Correlated binocular activity guides recovery from monocular deprivation. Nature 416, 430–433 (2002).
Adams, D. L., Sincich, L. C. & Horton, J. C. Complete pattern of ocular dominance columns in human primary visual cortex. J. Neurosci. 27, 10391–10403 (2007).
Kershman, J. The medulloblast and the medulloblastoma: a study of human embryos. Arch. Neurol. Psychiat. 40, 937–967 (1938).
Pollard, K. S. et al. An RNA gene expressed during cortical development evolved rapidly in humans. Nature 443, 167–172 (2006).
Metin, C., Denizot, J. P. & Ropert, N. Intermediate zone cells express calcium-permeable AMPA receptors and establish close contact with growing axons. J. Neurosci. 20, 696–708 (2000).
De Carlos, J. A. & O'Leary, D. D. Growth and targeting of subplate axons and establishment of major cortical pathways. J. Neurosci. 12, 1194–1211 (1992).
O'Leary, D. D. & Koester, S. E. Development of projection neuron types, axon pathways, and patterned connections of the mammalian cortex. Neuron 10, 991–1006 (1993).
Del Rio, J. A., Martinez, A., Auladell, C. & Soriano, E. Developmental history of the subplate and developing white matter in the murine neocortex. Neuronal organization and relationship with the main afferent systems at embryonic and perinatal stages. Cereb. Cortex 10, 784–801 (2000).
Molnar, Z., Adams, R. & Blakemore, C. Mechanisms underlying the early establishment of thalamocortical connections in the rat. J. Neurosci. 18, 5723–5745 (1998).
Takahashi, T., Nowakowski, R. S. & Caviness, V. S. Jr. The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall. J. Neurosci. 15, 6046–6057 (1995).
Ulfig, N., Neudorfer, F. & Bohl, J. Transient structures of the human fetal brain: subplate, thalamic reticular complex, ganglionic eminence. Histol. Histopathol. 15, 771–790 (2000).
Parnavelas, J. G. The origin and migration of cortical neurones: new vistas. Trends Neurosci. 23, 126–131 (2000).
Kostovic, I. & Rakic, P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J. Comp. Neurol. 297, 441–470 (1990).
Bystron, I., Blakemore C. & Rakic, P. Early development of the human cerebral cortex: formation of transient embryonic zones IBRO Abstr. s20070131004126 (2007).
Kostovic I. & Molliver, M. E. A new interpretation of the laminar development of cerebral cortex: synaptogenesis in different layers of neopallium in the human fetus. Anat. Rec. 178, 395 (1974). This paper, reported at the annual meeting of the American Association of Anatomists, was the first to mention the existence of the SP; the full-size paper, which contained a detailed description, was published 15 years later (see reference 107).
Rakic, P. Prenatal development of the visual system in rhesus monkey. Philos. Trans. R. Soc. Lond. B Biol. Sci. 278, 245–260 (1977).
Rakic, P. Early developmental events: cell lineages, acquisition of neuronal positions, and areal and laminar development. Neurosci. Res. Program Bull. 20, 439–451 (1982).
Luskin, M. B. & Shatz, C. J. Studies of the earliest generated cells of the cat's visual cortex: cogeneration of subplate and marginal zones. J. Neurosci. 5, 1062–1075 (1985).
Valverde, F., Facal-Valverde, M. V., Santacana, M. & Heredia, M. Development and differentiation of early generated cells of sublayer VIb in the somatosensory cortex of the rat: a correlated Golgi and autoradiographic study. J. Comp. Neurol. 290, 118–140 (1989).
Hevner, R. F., Daza, R. A., Englund, C., Kohtz, J. & Fink, A. Postnatal shifts of interneuron position in the neocortex of normal and reeler mice: evidence for inward radial migration. Neuroscience 124, 605–618 (2004).
McConnell, S. K., Ghosh, A. & Shatz, C. J. Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science 245, 978–982 (1989).
Kanold, P. O. Transient microcircuits formed by subplate neurons and their role in functional development of thalamocortical connections. Neuroreport 15, 2149–2153 (2004).
Chan, C. H. et al. Emx1 is a marker for pyramidal neurons of the cerebral cortex. Cereb. Cortex 11, 1191–1198 (2001).
Flames, N. et al. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron 44, 251–261 (2004).
Kostovic, I. & Goldman-Rakic, P. S. Transient cholinesterase staining in the mediodorsal nucleus of the thalamus and its connections in the developing human and monkey brain. J. Comp. Neurol. 219, 431–447 (1983).
Bystron, I., Blakemore, C., Otellin, V. & Molnar, Z. Early development of the thalamic reticular and perireticular nuclei in the human brain. J. Physiol. 548P, 144 (2003).
Rakic, P. Prenatal development of the visual system in the rhesus monkey. Philos. Trans. R. Soc. Lond. B Biol. Sci. 278, 245–260 (1973). This paper provided the first report, based on experiments in fetuses of non-human primates, that thalamic input arrives at the subplate zone early and 'waits' until the final target neurons have been generated and have assumed positions in layer 4 of the developing cortex.
Meinecke, D. L. & Rakic, P. Expression of GABA and GABAA receptors by neurons of the subplate zone in developing primate occipital cortex: evidence for transient local circuits. J. Comp. Neurol. 317, 91–101 (1992).
Kostovic, I., Judas, M., Rados, M. & Hrabac, P. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb. Cortex 12, 536–544 (2002).
Kostovic, I. et al. Structural basis of the developmental plasticity in the human cerebral cortex: the role of the transient subplate zone. Metab. Brain Dis. 4, 17–23 (1989).
Jimenez, D., Rivera, R., Lopez-Mascaraque, L. & De Carlos, J. A. Origin of the cortical layer I in rodents. Dev. Neurosci. 25, 105–115 (2003).
Zecevic, N. & Rakic, P. Development of layer I neurons in the primate cerebral cortex. J. Neurosci. 21, 5607–5619 (2001).
Brun, A. The subpial granular layer of the foetal cerebral cortex in man. Its ontogeny and significance in congenital cortical malformations. Acta Pathol. Microbiol. Scand. 179 (suppl.), 3–98 (1965).
Gadisseux, J. F., Goffinet, A. M., Lyon, G. & Evrard, P. The human transient subpial granular layer: an optical, immunohistochemical, and ultrastructural analysis. J. Comp. Neurol. 324, 94–114 (1992). This is a detailed study of the SG layers in the developing human cerebral cortex. These layers, although well described in the classical literature and confirmed in a monograph (see reference 127), are neglected in the literature because they have not been observed in rodents.
Meyer, G. & Goffinet, A. M. Prenatal development of reelin-immunoreactive neurons in the human neocortex. J. Comp. Neurol. 397, 29–40 (1998).
Meyer, G. & Gonzalez-Hernandez, T. Developmental changes in layer I of the human neocortex during prenatal life: a DiI-tracing and AChE and NADPH-d histochemistry study. J. Comp. Neurol. 338, 317–336 (1993).
Kostovic, I., Jovanov-Milosevic, N., Krsnik, Z., Petanjek, Z. & Judas, M. Laminar organization of the marginal zone in the human fetal cortex. Neuroembryol. Aging 3, 19–26 (2004).
Choi, B. H. Glial fibrillary acidic protein in radial glia of early human fetal cerebrum: a light and electron microscopic immunoperoxidase study. J. Neuropathol. Exp. Neurol. 45, 408–418 (1986).
Schmechel, D. E. & Rakic, P. Arrested proliferation of radial glial cells during midgestation in rhesus monkey. Nature 277, 303–305 (1979).
Allman, J., Hakeem, A. & Watson, K. Two phylogenetic specializations in the human brain. Neuroscientist 8, 335–346 (2002).
Preuss, T. M. Preface: from basic uniformity to diversity in cortical organization. Brain Behav. Evol. 55, 283–286 (2000).
Cholfin, J. A. & Rubenstein, J. L. Patterning of frontal cortex subdivisions by Fgf17. Proc. Natl Acad. Sci. USA 104, 7652–7657 (2007). This study provided experimental evidence that cytoarchitectonic areas are specified in the proliferative zones, as predicted by the protomap hypothesis (see reference 13). Together with references 6 and 28, this study also showed that the manipulation of cell proliferation and death rate in the VZ can independently change the size of a selected cortical area, providing a working model of the differential expansion of cytoarchitectonic maps during evolution
Krubitzer, L. & Kaas, J. The evolution of the neocortex in mammals: how is phenotypic diversity generated? Curr. Opin. Neurobiol. 15, 444–453 (2005).
Chenn, A. & Walsh, C. A. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297, 365–369 (2002).
Zhang, J. Evolution of the human ASPM gene, a major determinant of brain size. Genetics 165, 2063–2070 (2003).
Evans, P. D. et al. Adaptive evolution of ASPM, a major determinant of cerebral cortical size in humans. Hum. Mol. Genet. 13, 489–494 (2004).
Levitt, P. & Rakic, P. Immunoperoxidase localization of glial fibrillary acidic protein in radial glial cells and astrocytes of the developing rhesus monkey brain. J. Comp. Neurol. 193, 815–840 (1980).
Rakic, P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J. Comp. Neurol. 145, 61–83 (1972). This paper, together with references 133 and 143, presented evidence from a combination of Golgi staining and serial electron microscopy that postmitotic neurons in the large and convoluted primate cerebrum follow the increasingly elongated and curvilinear shafts of the radial glial cells, some of which do not divide while transiently serving as migratory guides.
Rakic, P. Guidance of neurons migrating to the fetal monkey neocortex. Brain Res. 33, 471–476 (1971).
Fishell, G. & Kriegstein, A. R. Neurons from radial glia: the consequences of asymmetric inheritance. Curr. Opin. Neurobiol. 13, 34–41 (2003). This article and reference 40 review the evidence that radial glial cells can produce neurons.
Noctor, S. C. et al. Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J. Neurosci. 22, 3161–3173 (2002).
Malatesta, P., Hartfuss, E. & Gotz, M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development 127, 5253–5263 (2000).
Temple, S. The development of neural stem cells. Nature 414, 112–117 (2001).
Retzius, G. Die Cajal'schen zellen der grosshirnrinde beim menschen und bei säugetieren. Biol. Untersuch. 5, 1–8 (1893).
Cajal, S. Sur la structure de lecorce cerebrale de quelques mammiferes. Cellule 7, 123–176 (1891).
Tissir, F. & Goffinet, A. M. Reelin and brain development. Nature Rev. Neurosci. 4, 496–505 (2003).
Meyer, G., Perez-Garcia, C. G., Abraham, H. & Caput, D. Expression of p73 and reelin in the developing human cortex. J. Neurosci. 22, 4973–4986 (2002).
Hevner, R. F., Neogi, T., Englund, C., Daza, R. A. & Fink, A. Cajal–Retzius cells in the mouse: transcription factors, neurotransmitters, and birthdays suggest a pallial origin. Brain Res. Dev. Brain Res. 141, 39–53 (2003).
Lavdas, A. A., Grigoriou, M., Pachnis, V. & Parnavelas, J. G. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J. Neurosci. 19, 7881–7888 (1999). This paper describes the origin of early-generated GABAergic interneurons and their tangential mode of migration from the medial portion of the ganglionic eminence of the ventral telencephalon to the cerebral cortex (see also reference 3 for a comprehensive review of this subject).
Meyer, G., Soria, J. M., Martinez-Galan, J. R., Martin-Clemente, B. & Fairen, A. Different origins and developmental histories of transient neurons in the marginal zone of the fetal and neonatal rat cortex. J. Comp. Neurol. 397, 493–518 (1998).
Cajal, S. Histologie du systeme nerveus de l'home et des vertebres. Reprinted by Consejo Superior de Investigaciones Cientificas (Paris, 1911).
Bystron, I., Rakic, P. & Blakemore, C. Early development of the cortical subplate in human and non-human primates. Abstr. - Soc. Neurosci. 674.10 (2007)
Acknowledgements
We are grateful to J. Rubenstein, R. Hevner, D. D. M. O'Leary, S. Lindsay and F. Guillemot for discussion about the IZ and the SP. This work was supported by the Kavli Institute for Neuroscience at Yale (P.R. & I.B.), the National Institute of Neurological Disorders and Stroke of the US Public Health Service (P.R.) and the Hill Foundation (I.B.)
Author information
Authors and Affiliations
Corresponding authors
Related links
Glossary
- Neocortex
-
The evolutionarily newest portion of the cerebral cortex. It is particularly enlarged in primates and underpins higher mental functions for humans.
- Neuroepithelium
-
A layer of proliferating neuroepithelial cells that makes up the neural plate and neural tube.
- Neural plate
-
The neuroectodermal epithelium before the neural groove and neural tube form.
- Interkinetic nuclear movement
-
The apical-basal-apical migration of the neuroepithelial cell nucleus during the cell cycle.
- Predecessor cell
-
A type of neuron that arrives beneath the pial surface of the human embryonic forebrain before the neural tube has even finished closing. Predecessor cells appear in the developing cortex before any other post-mitotic cells.
- Golgi staining
-
A staining technique introduced by Camillo Golgi in 1873 that selectively stains neurons with silver nitrate.
- Projection neuron
-
A type of glutamatergic neuron, characterized by a typical pyramidal morphology, that extends its axon to distant intracortical, subcortical or subcerebral targets. Projection neurons are born in the VZ, and their lineage is different from that of the interneurons.
- Interneurons
-
A heterogeneous group of non-pyramidal, mostly GABAergic neurons that project locally and appear to be mainly inhibitory. In rodents most interneurons originate in the ganglionic eminence of the ventral forebrain and then migrate tangentially to the neocortex, whereas in humans and non-human primates they originate from both the ganglionic eminence and the SVZ of the dorsal telencephalon. In the human brain there might be pronounced differences in the proportions of neurons from the two sources.
- Corticofugal axon
-
An axon that originates from a cortical neuron but projects ouside the cortex to subcortical structures.
- Internal capsule
-
A large bundle of axons that reciprocally connects the cortex with the subcortical structures of the brain.
Rights and permissions
About this article
Cite this article
Bystron, I., Blakemore, C. & Rakic, P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci 9, 110–122 (2008). https://doi.org/10.1038/nrn2252
Issue Date:
DOI: https://doi.org/10.1038/nrn2252
This article is cited by
-
Effect of breast milk intake volume on early behavioral neurodevelopment of extremely preterm infants
International Breastfeeding Journal (2024)
-
Modeling tuberous sclerosis complex with human induced pluripotent stem cells
World Journal of Pediatrics (2024)
-
Laminar dynamics of deep projection neurons and mode of subplate formation are hallmarks of histogenetic subdivisions of the human cingulate cortex before onset of arealization
Brain Structure and Function (2023)
-
Pan-cancer surveys indicate cell cycle-related roles of primate-specific genes in tumors and embryonic cerebrum
Genome Biology (2022)
-
Modeling human neurodevelopmental diseases with brain organoids
Cell Regeneration (2022)