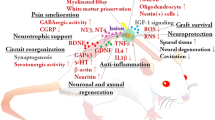
Key Points
-
Spinal cord injury (SCI) can lead to paraplegia or quadriplegia and is a devastating condition for which there is as yet no cure. Cellular and molecular, as well as rehabilitative training, therapies are being developed in animal models, and some are now in, or moving towards, clinical trials.
-
Cellular therapeutic interventions after SCI include transplantation of peripheral nerve cells, Schwann cells, cells from the olfactory nervous system, stem/progenitor cells and activated macrophages.
-
Molecular therapeutic interventions after SCI include neuroprotective therapies, axonal conduction enhancement, growth factor delivery, cyclic AMP delivery, small GTPase delivery, and therapies that modulate interactions with myelin inhibitors and extracellular matrix modifiers.
-
Some of these interventions are still at the animal experimental stage. However, there are interventions that are progressing to potential application in humans through the use of human cells in animal models (for example, transplantation of Schwann cells, and stem/progenitor cells). Some therapies are already in clinical trials to treat other diseases (for example, administration of nerve growth factor in the treatment of Alzheimer's disease).
-
Other potential therapies are already in, or progressing towards, clinical trials, including transplantation of cells from the olfactory nervous system, bone marrow stromal cells and activated macrophages. The potential of 4-aminopyridine, Cethrin and Nogo-A antibodies is also being tested.
-
Improved locomotor function can be achieved with rehabilitation, because the spinal circuitry below the lesion site maintains active and functional neuronal properties, such that it can generate oscillating, coordinated motor patterns and is capable of considerable plasticity. Many SCI clinical trials are currently addressing aspects of rehabilitation, including upper-extremity exercise, body-weight-supported treadmill training and/or functional electric stimulation.
-
However, much work remains to be done to determine whether any of these therapies can safely improve outcome after human SCI. To identify therapies that are unambiguously safe and effective, the scientific and clinical SCI communities increasingly recommend that preclinical studies should be reproduced by independent laboratories. Finally, individual therapies are unlikely to emerge as a sole cure. Rather, we predict that combinations of strategies will lead to collective improvement in outcome after SCI.
Abstract
Spinal cord injury (SCI) can lead to paraplegia or quadriplegia. Although there are no fully restorative treatments for SCI, various rehabilitative, cellular and molecular therapies have been tested in animal models. Many of these have reached, or are approaching, clinical trials. Here, we review these potential therapies, with an emphasis on the need for reproducible evidence of safety and efficacy. Individual therapies are unlikely to provide a panacea. Rather, we predict that combinations of strategies will lead to improvements in outcome after SCI. Basic scientific research should provide a rational basis for tailoring specific combinations of clinical therapies to different types of SCI.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Anderson, K. D. Targeting recovery: priorities of the spinal cord-injured population. J. Neurotrauma 21, 1371–1383 (2004). An important study that reveals the factors that patients with SCI consider to be the most important to enhance their quality of life.
Nash, M. S. Exercise as a health-promoting activity following spinal cord injury. J. Neurol. Phys. Ther. 29, 87–103, 106 (2005).
Widerstrom-Noga, E. G. & Turk, D. C. Types and effectiveness of treatments used by people with chronic pain associated with spinal cord injuries: influence of pain and psychosocial characteristics. Spinal Cord 41, 600–609 (2003).
Waxman, S. G. & Hains, B. C. Fire and phantoms after spinal cord injury: Na+ channels and central pain. Trends Neurosci. 29, 207–215 (2006).
Eaton, M. J. Cell and molecular approaches to the attenuation of pain after spinal cord injury. J. Neurotrauma 23, 549–559 (2006).
Kirshblum, S. Treatment alternatives for spinal cord injury related spasticity. J. Spinal Cord Med. 22, 199–217 (1999).
Kleitman, N. Keeping promises: translating basic research into new spinal cord injury therapies. J. Spinal Cord Med. 27, 311–318 (2004).
Amador, M. J. & Guest, J. D. An appraisal of ongoing experimental procedures in human spinal cord injury. J. Neurol. Phys. Ther. 29, 70–86 (2005).
Blight, A. R. & Tuszynski, M. H. Clinical trials in spinal cord injury. J. Neurotrauma 23, 586–593 (2006).
Anderson, D. K. et al. Recommended guidelines for studies of human subjects with spinal cord injury. Spinal Cord 43, 453–458 (2005).
Steeves, J., Fawcett, J. & Tuszynski, M. Report of international clinical trials workshop on spinal cord injury February 20–21, 2004, Vancouver, Canada. Spinal Cord 42, 591–597 (2004).
Hall, E. D. Drug development in spinal cord injury: what is the FDA looking for? J. Rehabil. Res. Dev. 40, 81–91 (2003).
Bunge, R. P., Puckett, W. R., Becerra, J. L., Marcillo, A. E. & Quencer, R. M. Observations on the pathology of human SCI — a review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv. Neurol. 59, 75–90 (1993).
Kakulas, B. A. A review of the neuropathology of human spinal cord injury with emphasis on special features. J. Spinal Cord Med. 22, 119–124 (1999).
Horky, L., Galimi, F., Gage, F. H. & Horner, P. J. Fate of endogenous stem/progenitor cells following spinal cord injury. J. Comp. Neurol. (in the press).
Crowe, M. J., Bresnahan, J. C., Shuman, S. L., Masters, J. N. & Beattie, M. S. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nature Med. 3, 73–76 (1997).
Guest, J. D., Hiester, E. D. & Bunge, R. P. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp. Neurol. 192, 384–393 (2005).
Totoiu, M. O. & Keirstead, H. S. Spinal cord injury is accompanied by chronic progressive demyelination. J. Comp. Neurol. 486, 373–383 (2005).
Jones, T. B., McDaniel, E. E. & Popovich, P. G. Inflammatory-mediated injury and repair in the traumatically injured spinal cord. Curr. Pharm. Des. 11, 1223–1236 (2005). Starts with an excellent primer explaining the body's innate and adaptive immune systems, and goes on to focus on the detrimental and potential reparative roles of neutrophils, macrophages, microglia, and T and B cells.
Jones, L. L., Yamaguchi, Y., Stallcup, W. B. & Tuszynski, M. H. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J. Neurosci. 22, 2792–2803 (2002).
Jones, L. L., Margolis, R. U. & Tuszynski, M. H. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp. Neurol. 182, 399–411 (2003).
Bruce, J. H. et al. Schwannosis: role of gliosis and proteoglycan in human spinal cord injury. J. Neurotrauma 17, 781–788 (2000).
Silver, J. & Miller, J. H. Regeneration beyond the glial scar. Nature Rev. Neurosci. 5, 146–156 (2004).
Fawcett, J. W. Overcoming inhibition in the damaged spinal cord. J. Neurotrauma 23, 371–383 (2006).
Beattie, M. S. et al. Endogenous repair after spinal cord contusion injuries in the rat. Exp. Neurol. 148, 453–463 (1997).
Yang, H. et al. Endogenous neurogenesis replaces oligodendrocytes and astrocytes after primate spinal cord injury. J. Neurosci. 26, 2157–2166 (2006).
Yamamoto, S., Yamamoto, N., Kitamura, T., Nakamura, K. & Nakafuku, M. Proliferation of parenchymal neural progenitors in response to injury in the adult rat spinal cord. Exp. Neurol. 172, 115–127 (2001).
Azari, M. F., Profyris, C., Zang, D. W., Petratos, S. & Cheema, S. S. Induction of endogenous neural precursors in mouse models of spinal cord injury and disease. Eur. J. Neurol. 12, 638–648 (2005).
Hill, C. E., Beattie, M. S. & Bresnahan, J. C. Degeneration and sprouting of identified descending supraspinal axons after contusive spinal cord injury in the rat. Exp. Neurol. 171, 153–169 (2001).
Pettigrew, D. B., Shockley, K. P. & Crutcher, K. A. Disruption of spinal cord white matter and sciatic nerve geometry inhibits axonal growth in vitro in the absence of glial scarring. BMC Neurosci. 2, 8 (2001).
Raineteau, O. & Schwab, M. E. Plasticity of motor systems after incomplete spinal cord injury. Nature Rev. Neurosci. 2, 263–273 (2001).
Weidner, N., Ner, A., Salimi, N. & Tuszynski, M. H. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc. Natl Acad. Sci. USA 98, 3513–3518 (2001).
Bareyre, F. M. et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nature Neurosci. 7, 269–277 (2004). Presents the important finding that significant spontaneous axonal sprouting occurs in the spinal cord after injury, which provides alternative routes through which corticospinal axons can activate lumbar neurons.
Raineteau, O., Fouad, K., Bareyre, F. M. & Schwab, M. E. Reorganization of descending motor tracts in the rat spinal cord. Eur. J. Neurosci. 16, 1761–1771 (2002).
Richardson, P. M., McGuinness, U. M. & Aguayo, A. J. Axons from CNS grafts regenerate into PNS grafts. Nature 284, 264–265 (1980).
Cheng, H., Cao, Y. & Olson, L. Spinal cord repair in adult paraplegic rats: partial restoration of hindlimb function. Science 273, 510–513 (1996).
Cheng, H. et al. Gait analysis of adult paraplegic rats after spinal cord repair. Exp. Neurol. 148, 544–557 (1997).
Lee, Y. S., Hsiao, I. & Lin, V. W. Peripheral nerve grafts and aFGF restore partial hindlimb function in adult paraplegic rats. J. Neurotrauma 19, 1203–1216 (2002).
Lee, Y. S., Lin, C. Y., Robertson, R. T., Hsiao, I. & Lin, V. W. Motor recovery and anatomical evidence of axonal regrowth in spinal cord-repaired adult rats. J. Neuropathol. Exp. Neurol. 63, 233–245 (2004).
Levi, A. D. et al. Peripheral nerve grafts promoting central nervous system regeneration after spinal cord injury in the primate. J. Neurosurg. 96, 197–205 (2002).
Cheng, H., Liao, K. K., Liao, S. F., Chuang, T. Y. & Shih, Y. H. Spinal cord repair with acidic fibroblast growth factor as a treatment for a patient with chronic paraplegia. Spine 29, E284–E288 (2004).
Takami, T. et al. Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J. Neurosci. 22, 6670–6681 (2002). An important study that compares two competing cell types in a single experiment.
Bamber, N. I. et al. Neurotrophins BDNF and NT-3 promote axonal re-entry into the distal host spinal cord through Schwann cell-seeded mini-channels. Eur. J. Neurosci. 13, 257–268 (2001).
Xu, X. M., Guenard, V., Kleitman, N. & Bunge, M. B. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J. Comp. Neurol. 351, 145–160 (1995).
Pinzon, A., Calancie, B., Oudega, M. & Noga, B. Conduction of impulses by axons regenerated in a Schwann cell graft in the transected adult rat spinal cord. J. Neurosci. Res. 64, 533–541 (2001).
Pearse, D. D. et al. Transplantation of Schwann cells and olfactory ensheathing glia after spinal cord injury: does pretreatment with methylprednisolone and interleukin-10 enhance recovery? J. Neurotrauma 21, 1223–1239 (2004).
Menei, P., Montero-Menei, C., Whittemore, S. R., Bunge, R. P. & Bunge, M. B. Schwann cells genetically modified to secrete human BDNF promote enhanced axonal regrowth across transected adult rat spinal cord. Eur. J. Neurosci. 10, 607–621 (1998).
Xu, X. M., Guenard, V., Kleitman, N., Aebischer, P. & Bunge, M. B. A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp. Neurol. 134, 261–272 (1995).
Chen, A., Xu, X. M., Kleitman, N. & Bunge, M. B. Methylprednisolone administration improves axonal regeneration into Schwann cell grafts in transected adult rat thoracic spinal cord. Exp. Neurol. 138, 261–276 (1996).
Ramon-Cueto, A., Plant, G. W., Avila, J. & Bunge, M. B. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J. Neurosci. 18, 3803–3815 (1998).
Guest, J. D. et al. The ability of cultured human Schwann cells within PAN/PVC guidance channels to support regeneration in the transected nude rat spinal cord. Can. J. Neurol. Sci. 23, S1–S2 (1996).
Iwanami, A. et al. Establishment of graded spinal cord injury model in a nonhuman primate: the common marmoset. J. Neurosci. Res. 80, 172–181 (2005).
Imaizumi, T., Lankford, K. L., Burton, W. V., Fodor, W. L. & Kocsis, J. D. Xenotransplantation of transgenic pig olfactory ensheathing cells promotes axonal regeneration in rat spinal cord. Nature Biotechnol. 18, 949–953 (2000).
Bianco, J. I., Perry, C., Harkin, D. G., Mackay-Sim, A. & Feron, F. Neurotrophin 3 promotes purification and proliferation of olfactory ensheathing cells from human nose. Glia 45, 111–123 (2004).
Kato, T., Honmou, O., Uede, T., Hashi, K. & Kocsis, J. D. Transplantation of human olfactory ensheathing cells elicits remyelination of demyelinated rat spinal cord. Glia 30, 209–218 (2000).
Barnett, S. C. et al. Identification of a human olfactory ensheathing cell that can effect transplant-mediated remyelination of demyelinated CNS axons. Brain 123, 1581–1588 (2000).
Casas, C. E. et al. Non-human primate model of autologous olfactory ensheathing glia transplantation following a stereotaxic electrolytic unilateral lesion of the medullary pyramid. Abstr. Soc. Neurosci. 184, 5 (2004).
Li, Y., Decherchi, P. & Raisman, G. Transplantation of olfactory ensheathing cells into spinal cord lesions restores breathing and climbing. J. Neurosci. 23, 727–731 (2003).
Lu, J., Feron, F., Ho, S. M., Mackay-Sim, A. & Waite, P. M. Transplantation of nasal olfactory tissue promotes partial recovery in paraplegic adult rats. Brain Res. 889, 344–357 (2001).
Li, Y., Field, P. M. & Raisman, G. Regeneration of adult rat corticospinal axons induced by transplanted olfactory ensheathing cells. J. Neurosci. 18, 10514–10524 (1998).
Li, Y., Field, P. M. & Raisman, G. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science 277, 2000–2002 (1997).
Ramon-Cueto, A., Cordero, M. I., Santos-Benito, F. F. & Avila, J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron 25, 425–435 (2000).
Plant, G. W., Christensen, C. L., Oudega, M. & Bunge, M. B. Delayed transplantation of olfactory ensheathing glia promotes sparing/regeneration of supraspinal axons in the contused adult rat spinal cord. J. Neurotrauma 20, 1–16 (2003).
Lu, J., Feron, F., Mackay-Sim, A. & Waite, P. M. Olfactory ensheathing cells promote locomotor recovery after delayed transplantation into transected spinal cord. Brain 125, 14–21 (2002).
Keyvan-Fouladi, N., Raisman, G. & Li, Y. Functional repair of the corticospinal tract by delayed transplantation of olfactory ensheathing cells in adult rats. J. Neurosci. 23, 9428–9434 (2003).
Sasaki, M., Hains, B. C., Lankford, K. L., Waxman, S. G. & Kocsis, J. D. Protection of corticospinal tract neurons after dorsal spinal cord transection and engraftment of olfactory ensheathing cells. Glia 53, 352–359 (2006).
Ruitenberg, M. J. et al. NT-3 expression from engineered olfactory ensheathing glia promotes spinal sparing and regeneration. Brain 128, 839–853 (2005).
Sasaki, M., Lankford, K. L., Zemedkun, M. & Kocsis, J. D. Identified olfactory ensheathing cells transplanted into the transected dorsal funiculus bridge the lesion and form myelin. J. Neurosci. 24, 8485–8493 (2004).
Boyd, J. G., Doucette, R. & Kawaja, M. D. Defining the role of olfactory ensheathing cells in facilitating axon remyelination following damage to the spinal cord. FASEB J. 19, 694–703 (2005). A thoughtful review that examines evidence for and against myelination by transplanted cells that are derived from the olfactory nervous system.
Riddell, J. S., Enriquez-Denton, M., Toft, A., Fairless, R. & Barnett, S. C. Olfactory ensheathing cell grafts have minimal influence on regeneration at the dorsal root entry zone following rhizotomy. Glia 47, 150–167 (2004).
Ramer, L. M., Richter, M. W., Roskams, A. J., Tetzlaff, W. & Ramer, M. S. Peripherally-derived olfactory ensheathing cells do not promote primary afferent regeneration following dorsal root injury. Glia 47, 189–206 (2004).
Steward, O. et al. A re-assessment of the consequences of delayed transplantation of olfactory lamina propria following complete spinal cord transection in rats. Exp. Neurol. 198, 483–499 (2006).
Huang, H. et al. Influence of patients' age on functional recovery after transplantation of olfactory ensheathing cells into injured spinal cord injury. Chin. Med. J. (Engl.) 116, 1488–1491 (2003).
Dobkin, B. H., Curt, A. & Guest, J. Cellular transplants in China: observational study from the largest human experiment in chronic spinal cord injury. Neurorehabil. Neural Repair 20, 5–13 (2006). Independent evaluation of participants who had been transplanted with cells from the fetal olfactory nervous system.
Guest, J., Herrera, L. P. & Qian, T. Rapid recovery of segmental neurological function in a tetraplegic patient following transplantation of fetal olfactory bulb-derived cells. Spinal Cord 44, 135–142 (2006).
Jeffery, N. D., Lakatos, A. & Franklin, R. J. Autologous olfactory glial cell transplantation is reliable and safe in naturally occurring canine spinal cord injury. J. Neurotrauma 22, 1282–1293 (2005). Describes safe veterinary intervention for SCI in dogs.
Feron, F. et al. Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain 128, 2951–2960 (2005).
Lakatos, A., Smith, P. M., Barnett, S. C. & Franklin, R. J. Meningeal cells enhance limited CNS remyelination by transplanted olfactory ensheathing cells. Brain 126, 598–609 (2003).
Murrell, W. et al. Multipotent stem cells from adult olfactory mucosa. Dev. Dyn. 233, 496–515 (2005).
Bregman, B. S., McAtee, M., Dai, H. N. & Kuhn, P. L. Neurotrophic factors increase axonal growth after spinal cord injury and transplantation in the adult rat. Exp. Neurol. 148, 475–494 (1997).
Jakeman, L. B. & Reier, P. J. Axonal projections between fetal spinal cord transplants and the adult rat spinal cord: a neuroanatomical tracing study of local interactions. J. Comp. Neurol. 307, 311–334 (1991).
Kunkel-Bagden, E. & Bregman, B. S. Spinal cord transplants enhance the recovery of locomotor function after spinal cord injury at birth. Exp. Brain Res. 81, 25–34 (1990).
Bregman, B. S. et al. Recovery of function after spinal cord injury: mechanisms underlying transplant-mediated recovery of function differ after spinal cord injury in newborn and adult rats. Exp. Neurol. 123, 3–16 (1993).
Reier, P. J., Stokes, B. T., Thompson, F. J. & Anderson, P. J. Fetal cell grafts into resection and contusion/compression injuries of the rat and cat spinal cord. Exp. Neurol. 115, 177–188 (1992).
Reier, P. J., Anderson, D. K., Young, W., Michel, M. E. & Fessler, R. Workshop on intraspinal transplantation and clinical application. J. Neurotrauma 11, 369–377 (1994).
Bregman, B. S. et al. Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury. Prog. Brain Res. 137, 257–273 (2002).
Coumans, J. V. et al. Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J. Neurosci. 21, 9334–9344 (2001).
Reier, P. J. Cellular transplantation strategies for spinal cord injury and translational neurobiology. NeuroRx 1, 424–451 (2004). Interesting review that describes in detail clinical trials that use cellular transplantation strategies after SCI. Results from some trials have yet to be published in peer-reviewed articles.
Falci, S. et al. Obliteration of a posttraumatic spinal cord cyst with solid human embryonic spinal cord grafts: first clinical attempt. J. Neurotrauma 14, 875–884 (1997).
Thompson, F. J. et al. Neurophysiological assessment of the feasibility and safety of neural tissue transplantation in patients with syringomyelia. J. Neurotrauma 18, 931–945 (2001).
McDonald, J. W. et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured spinal cord. Nature Med. 5, 1410–1412 (1999).
Harper, J. M. et al. Axonal growth of embryonic stem cell-derived motoneurons in vitro and in motoneuron-injured adult rats. Proc. Natl Acad. Sci. USA 101, 7123–7128 (2004).
Cao, Q. L., Howard, R. M., Dennison, J. B. & Whittemore, S. R. Differentiation of engrafted neuronal-restricted precursor cells is inhibited in the traumatically injured spinal cord. Exp. Neurol. 177, 349–359 (2002).
Herrera, J. et al. Embryonic-derived glial-restricted precursor cells (GRP cells) can differentiate into astrocytes and oligodendrocytes in vivo. Exp. Neurol. 171, 11–21 (2001).
Cao, Q., Benton, R. L. & Whittemore, S. R. Stem cell repair of central nervous system injury. J. Neurosci. Res. 68, 501–510 (2002).
Han, S. S., Kang, D. Y., Mujtaba, T., Rao, M. S. & Fischer, I. Grafted lineage-restricted precursors differentiate exclusively into neurons in the adult spinal cord. Exp. Neurol. 177, 360–375 (2002).
Han, S. S., Liu, Y., Tyler-Polsz, C., Rao, M. S. & Fischer, I. Transplantation of glial-restricted precursor cells into the adult spinal cord: survival, glial-specific differentiation, and preferential migration in white matter. Glia 45, 1–16 (2004).
Yan, J., Welsh, A. M., Bora, S. H., Snyder, E. Y. & Koliatsos, V. E. Differentiation and tropic/trophic effects of exogenous neural precursors in the adult spinal cord. J. Comp. Neurol. 480, 101–114 (2004).
Hill, C. E. et al. Acute transplantation of glial-restricted precursor cells into spinal cord contusion injuries: survival, differentiation, and effects on lesion environment and axonal regeneration. Exp. Neurol. 190, 289–310 (2004).
Ogawa, Y. et al. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J. Neurosci. Res. 69, 925–933 (2002).
Teng, Y. D. et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc. Natl Acad. Sci. USA 99, 3024–3029 (2002).
Cummings, B. J. et al. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc. Natl Acad. Sci. USA 102, 14069–14074 (2005). Important study in which transplanted cells are also ablated to determine whether they have a role in recovered function.
Iwanami, A. et al. Transplantation of human neural stem cells for spinal cord injury in primates. J. Neurosci. Res. 80, 182–190 (2005).
Mitsui, T., Shumsky, J. S., Lepore, A. C., Murray, M. & Fischer, I. Transplantation of neuronal and glial restricted precursors into contused spinal cord improves bladder and motor functions, decreases thermal hypersensitivity, and modifies intraspinal circuitry. J. Neurosci. 25, 9624–9636 (2005).
Keirstead, H. S. et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J. Neurosci. 25, 4694–4705 (2005).
Faulkner, J. & Keirstead, H. S. Human embryonic stem cell-derived oligodendrocyte progenitors for the treatment of spinal cord injury. Transpl. Immunol. 15, 131–142 (2005).
Schultz, S. S. Adult stem cell application in spinal cord injury. Curr. Drug Targets 6, 63–73 (2005).
Koshizuka, S. et al. Transplanted hematopoietic stem cells from bone marrow differentiate into neural lineage cells and promote functional recovery after spinal cord injury in mice. J. Neuropathol. Exp. Neurol. 63, 64–72 (2004).
Koda, M. et al. Hematopoietic stem cell and marrow stromal cell for spinal cord injury in mice. Neuroreport 16, 1763–1767 (2005).
Hofstetter, C. P. et al. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nature Neurosci. 8, 346–353 (2005).
Wu, S. et al. Bone marrow stromal cells enhance differentiation of cocultured neurosphere cells and promote regeneration of injured spinal cord. J. Neurosci. Res. 72, 343–351 (2003).
Reynolds, B. A. & Weiss, S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255, 1707–1710 (1992).
Gage, F. H. et al. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc. Natl Acad. Sci. USA 92, 11879–11883 (1995).
Shihabuddin, L. S., Horner, P. J., Ray, J. & Gage, F. H. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J. Neurosci. 20, 8727–8735 (2000).
Vroemen, M., Aigner, L., Winkler, J. & Weidner, N. Adult neural progenitor cell grafts survive after acute spinal cord injury and integrate along axonal pathways. Eur. J. Neurosci. 18, 743–751 (2003).
Cao, Q. L. et al. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp. Neurol. 167, 48–58 (2001).
Karimi-Abdolrezaee, S., Eftekharpour, E., Wang, J., Morshead, C. M. & Fehlings, M. G. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J. Neurosci. 26, 3377–3389 (2006).
Horner, P. J. et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J. Neurosci. 20, 2218–2228 (2000).
Howard, M. J., Liu, S., Schottler, F., Snider, B. J. & Jacquin, M. F. Transplantation of apoptosis-resistant embryonic stem cells into the injured rat spinal cord. Somatosens. Mot. Res. 22, 37–44 (2005).
Chen, J., Bernreuther, C., Dihne, M. & Schachner, M. Cell adhesion molecule L1-transfected embryonic stem cells with enhanced survival support regrowth of corticospinal tract axons in mice after spinal cord injury. J. Neurotrauma 22, 896–906 (2005).
Cao, Q. et al. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J. Neurosci. 25, 6947–6957 (2005).
Hamada, M. et al. Introduction of the MASH1 gene into mouse embryonic stem cells leads to differentiation of motoneuron precursors lacking Nogo receptor expression that can be applicable for transplantation to spinal cord injury. Neurobiol. Dis. 22, 509–522 (2006).
Hirschberg, D. L. & Schwartz, M. Macrophage recruitment to acutely injured central nervous system is inhibited by a resident factor: a basis for an immune-brain barrier. J. Neuroimmunol. 61, 89–96 (1995).
Rapalino, O. et al. Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nature Med. 4, 814–821 (1998).
Bomstein, Y. et al. Features of skin-coincubated macrophages that promote recovery from spinal cord injury. J. Neuroimmunol. 142, 10–16 (2003).
Moon, L. & Bunge, M. B. From animal models to humans: strategies for promoting CNS axon regeneration and recovery of limb function after spinal cord injury. J. Neurol. Phys. Ther. 29, 55–69 (2005).
Popovich, P. G. et al. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J. Neuropathol. Exp. Neurol. 61, 623–633 (2002).
Popovich, P. G. et al. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp. Neurol. 158, 351–365 (1999).
Knoller, N. et al. Clinical experience using incubated autologous macrophages as a treatment for complete spinal cord injury: Phase I study results. J. Neurosurg. Spine 3, 173–181 (2005).
Baptiste, D. C. & Fehlings, M. G. Pharmacological approaches to repair the injured spinal cord. J. Neurotrauma 23, 318–334 (2006).
Gris, D. et al. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J. Neurosci. 24, 4043–4051 (2004).
Lee, S. M. et al. Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J. Neurotrauma 20, 1017–1027 (2003).
Teng, Y. D. et al. Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury. Proc. Natl Acad. Sci. USA 101, 3071–3076 (2004).
Stirling, D. P. et al. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J. Neurosci. 24, 2182–2190 (2004).
Hall, E. D. & Springer, J. E. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx 1, 80–100 (2004).
Coleman, W. P. et al. A critical appraisal of the reporting of the National Acute Spinal Cord Injury Studies (II and III) of methylprednisolone in acute spinal cord injury. J. Spinal Disord. 13, 185–199 (2000).
Hurlbert, R. J. Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J. Neurosurg. 93, 1–7 (2000).
Fehlings, M. G. & Baptiste, D. C. Current status of clinical trials for acute spinal cord injury. Injury 36 (Suppl. 2), B113–B122 (2005).
Short, D. J., El Masry, W. S. & Jones, P. W. High dose methylprednisolone in the management of acute spinal cord injury — a systematic review from a clinical perspective. Spinal Cord 38, 273–286 (2000).
Bracken, M. B. Methylprednisolone and acute spinal cord injury: an update of the randomized evidence. Spine 26, S47–S54 (2001).
McTigue, D. M., Horner, P. J., Stokes, B. T. & Gage, F. H. Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J. Neurosci. 18, 5354–5365 (1998).
Houweling, D. A. et al. Local application of collagen containing brain-derived neurotrophic factor decreases the loss of function after spinal cord injury in the adult rat. Neurosci. Lett. 251, 193–196 (1998).
Jakeman, L. B., Wei, P., Guan, Z. & Stokes, B. T. Brain-derived neurotrophic factor stimulates hindlimb stepping and sprouting of cholinergic fibers after spinal cord injury. Exp. Neurol. 154, 170–184 (1998).
Namiki, J., Kojima, A. & Tator, C. H. Effect of brain-derived neurotrophic factor, nerve growth factor, and neurotrophin-3 on functional recovery and regeneration after spinal cord injury in adult rats. J. Neurotrauma 17, 1219–1231 (2000).
Shumsky, J. S. et al. Delayed transplantation of fibroblasts genetically modified to secrete BDNF and NT-3 into a spinal cord injury site is associated with limited recovery of function. Exp. Neurol. 184, 114–130 (2003).
Tobias, C. A. et al. Delayed grafting of BDNF and NT-3 producing fibroblasts into the injured spinal cord stimulates sprouting, partially rescues axotomized red nucleus neurons from loss and atrophy, and provides limited regeneration. Exp. Neurol. 184, 97–113 (2003).
Zhou, L. & Shine, H. D. Neurotrophic factors expressed in both cortex and spinal cord induce axonal plasticity after spinal cord injury. J. Neurosci. Res. 74, 221–226 (2003).
Lee, T. T., Green, B. A., Dietrich, W. D. & Yezierski, R. P. Neuroprotective effects of basic fibroblast growth factor following spinal cord contusion injury in the rat. J. Neurotrauma 16, 347–356 (1999).
Blesch, A. & Tuszynski, M. H. Cellular GDNF delivery promotes growth of motor and dorsal column sensory axons after partial and complete spinal cord transections and induces remyelination. J. Comp. Neurol. 467, 403–417 (2003).
Tuszynski, M. H., Grill, R., Jones, L. L., McKay, H. M. & Blesch, A. Spontaneous and augmented growth of axons in the primate spinal cord: effects of local injury and nerve growth factor-secreting cell grafts. J. Comp. Neurol. 449, 88–101 (2002).
Tuszynski, M. H. et al. NT-3 gene delivery elicits growth of chronically injured corticospinal axons and modestly improves functional deficits after chronic scar resection. Exp. Neurol. 181, 47–56 (2003).
Mitsui, T., Fischer, I., Shumsky, J. S. & Murray, M. Transplants of fibroblasts expressing BDNF and NT-3 promote recovery of bladder and hindlimb function following spinal contusion injury in rats. Exp. Neurol. 194, 410–431 (2005).
Blesch, A., Yang, H., Weidner, N., Hoang, A. & Otero, D. Axonal responses to cellularly delivered NT-4/5 after spinal cord injury. Mol. Cell. Neurosci. 27, 190–201 (2004).
Schnell, L., Schneider, R., Kolbeck, R., Barde, Y. A. & Schwab, M. E. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesions. Nature 367, 170–172 (1994).
Zhou, L., Baumgartner, B. J., Hill-Felberg, S. J., McGowen, L. R. & Shine, H. D. Neurotrophin-3 expressed in situ induces axonal plasticity in the adult injured spinal cord. J. Neurosci. 23, 1424–1431 (2003).
Koda, M. et al. Adenovirus vector-mediated in vivo gene transfer of brain-derived neurotrophic factor (BDNF) promotes rubrospinal axonal regeneration and functional recovery after complete transection of the adult rat spinal cord. J. Neurotrauma 21, 329–337 (2004).
Blits, B. & Bunge, M. B. Direct gene therapy for repair of the spinal cord. J. Neurotrauma 23, 508–520 (2006). Thorough review of viral vectors that have been used to deliver molecules of interest in the context of SCI.
Grill, R., Blesch, A. & Tuszynski, M. H. Robust growth of chronically injured spinal cord axons induced by grafts of genetically modified NGF-secreting cells. Exp. Neurol. 148, 444–452 (1997).
Tuszynski, M. H. et al. Nerve growth factor delivery by gene transfer induces differential outgrowth of sensory, motor and noradrenergic neurites after adult spinal cord injury. Exp. Neurol. 137, 157–173 (1996).
Grill, R., Murai, K., Blesch, A., Gage, F. H. & Tuszynski, M. H. Cellular delivery of neurotrophin-3 promotes corticospinal axon growth and partial functional recovery after spinal cord injury. J. Neurosci. 17, 5560–5572 (1997).
Jin, Y., Fischer, I., Tessler, A. & Houle, J. D. Transplants of fibroblasts genetically modified to express BDNF promote axonal regeneration from supraspinal neurons following chronic spinal cord injury. Exp. Neurol. 177, 265–275 (2002).
Jin, Y., Tessler, A., Fischer, I. & Houle, J. D. Fibroblasts genetically modified to produce BDNF support regrowth of chronically injured serotonergic axons. Neurorehabil. Neural Repair 14, 311–317 (2000).
Liu, Y. et al. Transplants of fibroblasts genetically modified to express BDNF promote regeneration of adult rat rubrospinal axons and recovery of forelimb function. J. Neurosci. 19, 4370–4387 (1999).
Lu, P., Blesch, A. & Tuszynski, M. H. Neurotrophism without neurotropism: BDNF promotes survival but not growth of lesioned corticospinal neurons. J. Comp. Neurol. 436, 456–470 (2001).
Lu, P., Jones, L. L. & Tuszynski, M. H. BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp. Neurol. 191, 344–360 (2005).
Nothias, J. M. et al. Combined effects of neurotrophin secreting transplants, exercise, and serotonergic drug challenge improve function in spinal rats. Neurorehabil. Neural Repair 19, 296–312 (2005).
Kwon, B. K. et al. Rubrospinal neurons fail to respond to brain-derived neurotrophic factor applied to the spinal cord injury site 2 months after cervical axotomy. Exp. Neurol. 189, 45–57 (2004).
Apfel, S. C. Nerve growth factor for the treatment of diabetic neuropathy: what went wrong, what went right, and what does the future hold? Int. Rev. Neurobiol. 50, 393–413 (2002).
Tuszynski, M. H. et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nature Med. 11, 551–555 (2005).
Cai, D. et al. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J. Neurosci. 21, 4731–4739 (2001).
Neumann, S., Bradke, F., Tessier-Lavigne, M. & Basbaum, A. I. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron 34, 885–893 (2002).
Kao, H. T. et al. A protein kinase A-dependent molecular switch in synapsins regulates neurite outgrowth. Nature Neurosci. 5, 431–437 (2002).
Qiu, J. et al. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron 34, 895–903 (2002).
Lu, P., Yang, H., Jones, L. L., Filbin, M. T. & Tuszynski, M. H. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J. Neurosci. 24, 6402–6409 (2004).
Bhatt, D. H., Otto, S. J., Depoister, B. & Fetcho, J. R. Cyclic AMP-induced repair of zebrafish spinal circuits. Science 305, 254–258 (2004).
Nikulina, E., Tidwell, J. L., Dai, H. N., Bregman, B. S. & Filbin, M. T. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc. Natl Acad. Sci. USA 101, 8786–8790 (2004).
Pearse, D. D. et al. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nature Med. 10, 610–616 (2004).
Niederost, B., Oertle, T., Fritsche, J., McKinney, R. A. & Bandtlow, C. E. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J. Neurosci. 22, 10368–10376 (2002).
Jain, A., Brady-Kalnay, S. M. & Bellamkonda, R. V. Modulation of Rho GTPase activity alleviates chondroitin sulfate proteoglycan-dependent inhibition of neurite extension. J. Neurosci. Res. 77, 299–307 (2004).
Schweigreiter, R. et al. Versican V2 and the central inhibitory domain of Nogo-A inhibit neurite growth via p75NTR/NgR-independent pathways that converge at RhoA. Mol. Cell. Neurosci. 27, 163–174 (2004).
Dergham, P. et al. Rho signaling pathway targeted to promote spinal cord repair. J. Neurosci. 22, 6570–6577 (2002).
Fournier, A. E., Takizawa, B. T. & Strittmatter, S. M. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J. Neurosci. 23, 1416–1423 (2003).
Sung, J. K. et al. A possible role of RhoA/Rho-kinase in experimental spinal cord injury in rat. Brain Res. 959, 29–38 (2003).
McKerracher, L. & Higuchi, H. Targeting Rho to stimulate repair after spinal cord injury. J. Neurotrauma 23, 309–317 (2006).
Chan, C. C. et al. Dose-dependent beneficial and detrimental effects of ROCK inhibitor Y27632 on axonal sprouting and functional recovery after rat spinal cord injury. Exp. Neurol. 196, 352–364 (2005).
Mueller, B. K., Mack, H. & Teusch, N. Rho kinase, a promising drug target for neurological disorders. Nature Rev. Drug Discov. 4, 387–398 (2005). Good review on the potential of ROCK inhibitors to prevent neurodegeneration and stimulate neuroregeneration in various neurological disorders, including SCI.
Hara, M. et al. Protein kinase inhibition by fasudil hydrochloride promotes neurological recovery after spinal cord injury in rats. J. Neurosurg. 93, 94–101 (2000).
Tanaka, H. et al. Cytoplasmic p21(Cip1/WAF1) enhances axonal regeneration and functional recovery after spinal cord injury in rats. Neuroscience 127, 155–164 (2004).
Wei, L. et al. Rho kinases play an obligatory role in vertebrate embryonic organogenesis. Development 128, 2953–2962 (2001).
Niederöst, B. P., Zimmermann, D. R., Schwab, M. E. & Bandtlow, C. E. Bovine CNS myelin contains neurite growth-inhibitory activity associated with chondroitin sulfate proteoglycans. J. Neurosci. 19, 8979–8989 (1999).
Schwab, M. E. Nogo and axon regeneration. Curr. Opin. Neurobiol. 14, 118–124 (2004).
Fouad, K., Klusman, I. & Schwab, M. E. Regenerating corticospinal fibers in the Marmoset (Callitrix jacchus) after spinal cord lesion and treatment with the anti-Nogo-A antibody IN-1. Eur. J. Neurosci. 20, 2479–2482 (2004).
Benson, M. D. et al. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc. Natl Acad. Sci. USA 102, 10694–10699 (2005).
Schnell, L. & Schwab, M. E. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature 343, 269–272 (1990).
Schnell, L. & Schwab, M. E. Sprouting and regeneration of lesioned corticospinal tract fibers in the adult rat spinal cord. Eur. J. Neurosci. 5, 1156–1171 (1993).
Bregman, B. S. et al. Recovery from spinal cord injury by antibodies to neurite growth inhibitors. Nature 378, 498–501 (1995).
von Meyenburg, J., Brosamle, C., Metz, G. A. & Schwab, M. E. Regeneration and sprouting of chronically injured corticospinal tract fibers in adult rats promoted by NT-3 and the mAb IN-1, which neutralizes myelin-associated neurite growth inhibitors. Exp. Neurol. 154, 583–594 (1998).
Thallmair, M. et al. Neurite growth inhibitors restrict plasticity and functional recovery following corticospinal tract lesions. Nature Neurosci. 1, 124–131 (1998).
Z'Graggen, W. J., Metz, G. A. S., Kaitje, G. L., Thallmair, M. & Schwab, M. E. Functional recovery and enhanced corticofugal plasticity after unilateral pyramidal tract lesion and blockade of myelin-associated neurite growth inhibitors in adult rats. J. Neurosci. 18, 4744–4757 (1998).
Oudega, M. et al. Neutralizing antibodies against neurite growth inhibitor ni-35/250 do not promote regeneration of sensory axons in the adult rat spinal cord. Neuroscience 100, 873–883 (2000).
Guest, J. D. et al. Influence of IN-1 antibody and acidic FGF-fibrin glue on the response of injured corticospinal tract axons to human Schwann cell grafts. J. Neurosci. Res. 50, 888–905 (1997).
Merkler, D. et al. Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-A. J. Neurosci. 21, 3665–3673 (2001).
Mandemakers, W. J. & Barres, B. A. Axon regeneration: it's getting crowded at the gates of TROY. Curr. Biol. 15, R302–R305 (2005).
Song, X. Y., Zhong, J. H., Wang, X. & Zhou, X. F. Suppression of p75NTR does not promote regeneration of injured spinal cord in mice. J. Neurosci. 24, 542–546 (2004).
Kim, J. E., Li, S., GrandPre, T., Qiu, D. & Strittmatter, S. M. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron 38, 187–199 (2003).
Simonen, M. et al. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron 38, 201–211 (2003).
Zheng, B. et al. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron 38, 213–224 (2003).
Zheng, B. et al. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc. Natl Acad. Sci. USA 102, 1205–1210 (2005).
Li, S. et al. Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J. Neurosci. 24, 10511–10520 (2004).
Ji, B. et al. Effect of combined treatment with methylprednisolone and soluble Nogo-66 receptor after rat spinal cord injury. Eur. J. Neurosci. 22, 587–594 (2005).
Li, S. & Strittmatter, S. M. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J. Neurosci. 23, 4219–4227 (2003).
GrandPre, T., Li, S. & Strittmatter, S. M. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature 417, 547–551 (2002).
Stichel, C. C. et al. Inhibition of collagen IV deposition promotes regeneration of CNS axons. Eur. J. Neurosci. 11, 632–646 (1999).
Klapka, N. et al. Suppression of fibrous scarring in spinal cord injury of rat promotes long-distance regeneration of corticospinal tract axons, rescue of primary motoneurons in somatosensory cortex and significant functional recovery. Eur. J. Neurosci. 22, 3047–3058 (2005).
Weidner, N., Grill, R. J. & Tuszynski, M. H. Elimination of basal lamina and the collagen 'scar' after spinal cord injury fails to augment corticospinal tract regeneration. Exp. Neurol. 160, 40–50 (1999).
Moon, L. D., Asher, R. A., Rhodes, K. E. & Fawcett, J. W. Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nature Neurosci. 4, 465–466 (2001).
Bradbury, E. J. et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416, 636–640 (2002).
Yick, L. W., Wu, W., So, K. F., Yip, H. K. & Shum, D. K. Chondroitinase ABC promotes axonal regeneration of Clarke's neurons after spinal cord injury. Neuroreport 11, 1063–1067 (2000).
Chau, C. H. et al. Chondroitinase ABC enhances axonal regrowth through Schwann cell-seeded guidance channels after spinal cord injury. FASEB J. 18, 194–196 (2004).
Fouad, K. et al. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J. Neurosci. 25, 1169–1178 (2005).
Caggiano, A. O., Zimber, M. P., Ganguly, A., Blight, A. R. & Gruskin, E. A. Chondroitinase ABCI improves locomotion and bladder function following contusion injury of the rat spinal cord. J. Neurotrauma 22, 226–239 (2005).
Engesser-Cesar, C., Anderson, A. J., Basso, D. M., Edgerton, V. R. & Cotman, C. W. Voluntary wheel running improves recovery from a moderate spinal cord injury. J. Neurotrauma 22, 157–171 (2005).
Ichiyama, R. M., Gerasimenko, Y. P., Zhong, H., Roy, R. R. & Edgerton, V. R. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci. Lett. 383, 339–344 (2005).
Bouyer, L. J. Animal models for studying potential training strategies in persons with spinal cord injury. J. Neurol. Phys. Ther. 29, 117–125 (2005).
Curt, A., Schwab, M. E. & Dietz, V. Providing the clinical basis for new interventional therapies: refined diagnosis and assessment of recovery after spinal cord injury. Spinal Cord 42, 1–6 (2004).
Edgerton, V. R., Kim, S. J., Ichiyama, R. M., Gerasimenko, Y. P. & Roy, R. R. Rehabilitative therapies after spinal cord injury. J. Neurotrauma 23, 560–570 (2006). Excellent review on locomotor rehabilitation after SCI that includes a comprehensive section on automaticity and plasticity of the spinal circuitry.
Edgerton, V. R., Tillakaratne, N. J., Bigbee, A. J., de Leon, R. D. & Roy, R. R. Plasticity of the spinal neural circuitry after injury. Annu. Rev. Neurosci. 27, 145–167 (2004).
Moon, L. D. F., Leasure, J. L., Gage, F. H. & Bunge, M. B. Environmental enrichment sustains locomotor function recovered following spinal cord transection and glial transplantation. Restor. Neurol. Neurosci. (in the press).
Lankhorst, A. J. et al. Effects of enriched housing on functional recovery after spinal cord contusive injury in the adult rat. J. Neurotrauma 18, 203–215 (2001).
Hutchinson, K. J., Gomez-Pinilla, F., Crowe, M. J., Ying, Z. & Basso, D. M. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain 127, 1403–1414 (2004).
Van Meeteren, N. L., Eggers, R., Lankhorst, A. J., Gispen, W. H. & Hamers, F. P. Locomotor recovery after spinal cord contusion injury in rats is improved by spontaneous exercise. J. Neurotrauma 20, 1029–1037 (2003).
Barbeau, H., Nadeau, S. & Garneau, C. Physical determinants, emerging concepts, and training approaches in gait of individuals with spinal cord injury. J. Neurotrauma 23, 571–585 (2006).
Field-Fote, E. C., Lindley, S. D. & Sherman, A. L. Locomotor training approaches for individuals with spinal cord injury: a preliminary report of walking-related outcomes. J. Neurol. Phys. Ther. 29, 127–137 (2005).
Wolpaw, J. R. Treadmill training after spinal cord injury: good but not better. Neurology 66, 466–467 (2006).
Dobkin, B. et al. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology 66, 484–493 (2006).
Herman, R., He, J., D'Luzansky, S., Willis, W. & Dilli, S. Spinal cord stimulation facilitates functional walking in a chronic, incomplete spinal cord injured. Spinal Cord 40, 65–68 (2002).
Wernig, A. Long-term body-weight supported treadmill training and subsequent follow-up in persons with chronic SCI: effects on functional walking ability and measures of subjective well-being. Spinal Cord 43, 291–298 (2005).
Humm, J. L., Kozlowski, D. A., James, D. C., Gotts, J. E. & Schallert, T. Use-dependent exacerbation of brain damage occurs during an early post-lesion vulnerable period. Brain Res. 783, 286–292 (1998).
de Leon, R. D., Hodgson, J. A., Roy, R. R. & Edgerton, V. R. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J. Neurophysiol. 79, 1329–1340 (1998).
Lu, J. & Ashwell, K. Olfactory ensheathing cells: their potential use for repairing the injured spinal cord. Spine 27, 887–892 (2002).
Morrissey, T. K., Kleitman, N. & Bunge, R. P. Isolation and functional characteristics of Schwann cells derived from adult peripheral nerve. J. Neurosci. 11, 2433–2442 (1991).
Emery, E., Li, X., Brunschwig, J. P., Olson, L. & Levi, A. D. Assessment of the malignant potential of mitogen stimulated human Schwann cells. J. Peripher. Nerv. Syst. 4, 107–116 (1999).
Lepore, A. C., Bakshi, A., Swanger, S. A., Rao, M. S. & Fischer, I. Neural precursor cells can be delivered into the injured cervical spinal cord by intrathecal injection at the lumbar cord. Brain Res. 1045, 206–216 (2005).
Bakshi, A. et al. Lumbar puncture delivery of bone marrow stromal cells in spinal cord contusion: a novel method for minimally invasive cell transplantation. J. Neurotrauma 23, 55–65 (2006).
Ramer, L. M. et al. Peripheral olfactory ensheathing cells reduce scar and cavity formation and promote regeneration after spinal cord injury. J. Comp. Neurol. 473, 1–15 (2004).
Hill, C. E., Moon, L. D., Wood, P. M. & Bunge, M. B. Labeled Schwann cell transplantation: cell loss, host Schwann cell replacement, and strategies to enhance survival. Glia 53, 338–343 (2006).
Barakat, D. J. et al. Survival, integration, and axon growth support of glia transplanted into the chronically contused spinal cord. Cell Transplant. 14, 225–240 (2005).
Hendriks, W. T., Ruitenberg, M. J., Blits, B., Boer, G. J. & Verhaagen, J. Viral vector-mediated gene transfer of neurotrophins to promote regeneration of the injured spinal cord. Prog. Brain Res. 146, 451–476 (2004).
Kaspar, B. K. et al. Adeno-associated virus effectively mediates conditional gene modification in the brain. Proc. Natl Acad. Sci. USA 99, 2320–2325 (2002).
Pirozzi, M. et al. Intramuscular viral delivery of paraplegin rescues peripheral axonopathy in a model of hereditary spastic paraplegia. J. Clin. Invest. 116, 202–208 (2006).
Bresnahan, J. C. An electron-microscopic analysis of axonal alterations following blunt contusion of the spinal cord of the rhesus monkey (Macaca mulatta). J. Neurol. Sci. 37, 59–82 (1978).
Bresnahan, J. C., King, J. S., Martin, G. F. & Yashon, D. A neuroanatomical analysis of spinal cord injury in the rhesus monkey (Macaca mulatta). J. Neurol. Sci. 28, 521–542 (1976).
Courtine, G. et al. Performance of locomotion and foot grasping following a unilateral thoracic corticospinal tract lesion in monkeys (Macaca mulatta). Brain 128, 2338–2358 (2005).
Hagg, T. & Oudega, M. Degenerative and spontaneous regenerative processes after spinal cord injury. J. Neurotrauma 23, 263–280 (2006).
Richter, M. W., Fletcher, P. A., Liu, J., Tetzlaff, W. & Roskams, A. J. Lamina propria and olfactory bulb ensheathing cells exhibit differential integration and migration and promote differential axon sprouting in the lesioned spinal cord. J. Neurosci. 25, 10700–10711 (2005).
Radojicic, M., Reier, P. J., Steward, O. & Keirstead, H. S. Septations in chronic spinal cord injury cavities contain axons. Exp. Neurol. 196, 339–341 (2005).
Ye, J. H. & Houle, J. D. Treatment of the chronically injured spinal cord with neurotrophic factors can promote axonal regeneration from supraspinal neurons. Exp. Neurol. 143, 70–81 (1997).
Kwon, B. K. et al. Survival and regeneration of rubrospinal neurons 1 year after spinal cord injury. Proc. Natl Acad. Sci. USA 99, 3246–3251 (2002).
Pittenger, M. F. et al. Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 (1999).
Punzel, M. & Ho, A. D. Divisional history and pluripotency of human hematopoietic stem cells. Ann. NY Acad. Sci. 938, 72–81 (2001).
Lee, J. et al. Migration and differentiation of nuclear fluorescence-labeled bone marrow stromal cells after transplantation into cerebral infarct and spinal cord injury in mice. Neuropathology 23, 169–180 (2003).
Woodbury, D., Schwarz, E. J., Prockop, D. J. & Black, I. B. Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 61, 364–370 (2000).
Mezey, E., Chandross, K. J., Harta, G., Maki, R. A. & McKercher, S. R. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science 290, 1779–1782 (2000).
Brazelton, T. R., Rossi, F. M., Keshet, G. I. & Blau, H. M. From marrow to brain: expression of neuronal phenotypes in adult mice. Science 290, 1775–1779 (2000).
Castro, R. F. et al. Failure of bone marrow cells to transdifferentiate into neural cells in vivo. Science 297, 1299 (2002).
Wurmser, A. E. & Gage, F. H. Stem cells: cell fusion causes confusion. Nature 416, 485–487 (2002).
Terada, N. et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 416, 542–545 (2002).
Camargo, F. D., Chambers, S. M. & Goodell, M. A. Stem cell plasticity: from transdifferentiation to macrophage fusion. Cell Prolif. 37, 55–65 (2004).
Lu, P. & Tuszynski, M. H. Can bone marrow-derived stem cells differentiate into functional neurons? Exp. Neurol. 193, 273–278 (2005).
Hofstetter, C. P. et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc. Natl Acad. Sci. USA 99, 2199–2204 (2002).
Svendsen, C. N., Bhattacharyya, A. & Tai, Y. T. Neurons from stem cells: preventing an identity crisis. Nature Rev. Neurosci. 2, 831–834 (2001).
Cogle, C. R. et al. Bone marrow transdifferentiation in brain after transplantation: a retrospective study. Lancet 363, 1432–1437 (2004).
Deng, J., Petersen, B. E., Steindler, D. A., Jorgensen, M. L. & Laywell, E. D. Mesenchymal stem cells spontaneously express neural proteins in culture, and are neurogenic after transplantation. Stem Cells 24, 1054–1064 (2006).
Jendelova, P. et al. Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J. Neurosci. Res. 76, 232–243 (2004).
Shier, D. N. Hole's Human Anatomy and Physiology. 7th edn (TM Higher Education Group, New York, 1996).
Acknowledgements
We thank the Christopher Reeve Foundation and the members of their International Research Consortium. S.T. and L.D.F.M. were Associate members of this Consortium while working in the laboratories of F.H.G. and M. B. Bunge, respectively. We also thank M. L. Gage for editing the manuscript. Discussion of potential therapies in this review does not constitute endorsement by the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
F.G. is a member of the Scientific Advisory Boards of and holds stock in: Ceregene, Inc., Stem Cells, Inc. and Brain Cells, Inc. S.T. and L.M. have no real or perceived competing financial interests.
Related links
Related links
FURTHER INFORMATION
American Spinal Injury Association
Foundation for Spinal Cord Injury Prevention, Care and Cure
International Campaign for Cures of Spinal Cord Injury Paralysis
Miami Project to Cure Paralysis (FORE-SCI)
NINDS workshop on translating promising strategies for spinal cord injury therapy
Reeve–Irvine Research Centre, University of California, Irvine (FORE-SCI)
Glossary
- Autonomic dysreflexia
-
A life-threatening condition defined as a sudden and severe increase in blood pressure and a simultaneous decrease in heart rate induced by a noxious stimulus below the level of injury. Individuals with an injury at T6 or above are at risk of developing this condition.
- Apoptosis
-
Controlled cell death, regulated by an intracellular programme of events.
- Trabeculae
-
Strands of connective tissue that project into cysts or cavities.
- Plasticity
-
Refers to adaptive changes in neurological function. The substrate could be anatomical (for example, collateral sprouting) or physiological (for example, changes in balance between inhibitory and excitatory transmission).
- Autologous transplants
-
A transplant is autologous if the recipient also serves as the donor.
- Olfactory lamina propria
-
A thick vascularized layer of connective tissue beneath the olfactory epithelium that contains nerve fibres wrapped by ensheathing glia.
- Phase I clinical trial
-
A clinical trial that includes a small number of human participants to determine the safety of a new drug or invasive medical device; allows the determination of drug dosage or toxicity limits.
- Phase II clinical trial
-
A clinical trial that includes a larger number of human participants than a Phase I trial and is intended to evaluate the efficacy of a treatment; side effects are also monitored.
- Allodynia
-
A type of neuropathic pain that manifests as increased sensitivity to normally innocuous stimuli.
- Phase III clinical trial
-
A clinical trial that includes high numbers of human participants to test a treatment or drug that has been shown to be efficacious with tolerable side effects in Phase I and Phase II clinical trials.
- GTPases
-
Small enzymes that interact with GTP, a molecule that is used as a source of cellular energy.
- Chondroitin sulphate
-
A glycosaminoglycan that can limit the growth of axons.
- NgR(310)ecto-Fc
-
Soluble function-blocking protein made by fusing part of the ectodomain of NgR to rat IgG1 Fc. It could act by providing a decoy, non-signalling receptor that competes for binding of myelin-associated inhibitors of axon growth.
- NEP1–40
-
Nogo-A has two inhibitory regions; amino-Nogo and Nogo-66. NEP1–40 is a peptide derived from Nogo-66 that antagonizes stimulation of NgR1 in vitro.
- Chondroitinase ABC
-
A bacterial enzyme that degrades chondroitin sulphate.
- Functional electrical stimulation
-
(FES). FES involves electrophysiological stimulation of spinal cord or peripheral nerves or muscle.
Rights and permissions
About this article
Cite this article
Thuret, S., Moon, L. & Gage, F. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci 7, 628–643 (2006). https://doi.org/10.1038/nrn1955
Issue Date:
DOI: https://doi.org/10.1038/nrn1955
This article is cited by
-
CD44-targeting hyaluronic acid-selenium nanoparticles boost functional recovery following spinal cord injury
Journal of Nanobiotechnology (2024)
-
Reprogramming of astrocytes to neuronal-like cells in spinal cord injury: a systematic review
Spinal Cord (2024)
-
FUNDC1-induced mitophagy protects spinal cord neurons against ischemic injury
Cell Death Discovery (2024)
-
Rodent Models of Spinal Cord Injury: From Pathology to Application
Neurochemical Research (2023)
-
Effect of multifactorial therapeutic approach on axonal regeneration and cell viability in an in-vitro model of spinal-derived neural injury
Cell and Tissue Banking (2023)