Key Points
-
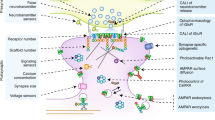
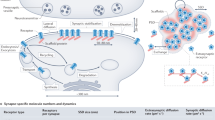
Long-term changes in synaptic efficacy commonly require gene expression and protein translation. However, this type of plastic change can be restricted to just a few of the contacts that a given neuron receives. How can cell-wide processes such as transcription be restricted to a small subset of synapses? An idea that has been put forward to answer this question is that of the 'synaptic tag', which marks synapses that are to experience the plastic change such that only those contacts with the tag can use the products of transcription.
-
Studies in Aplysia cell cultures and rat hippocampal slices have provided good evidence for the existence of a synaptic tag. In both cases, once a neuron has been stimulated to undergo long-term, transcription-dependent synaptic plasticity, the cell can use stimuli that normally produce transient changes in synaptic strength to induce more persistent changes at a different set of synapses. This capability persists only within a discrete time window, during which the putative tag can capture the products of gene expression that are induced by strong activity elsewhere in the neuron.
-
Any candidate synaptic tag should fulfil several criteria. First, it should be spatially restricted. Second, it should be time-limited and reversible. Third, it should be able to interact with cell-wide molecular events that occur after strong stimulation. So, from a broad perspective, anything that provides a spatially restricted trace of activity is a candidate synaptic tag.
-
Persistently active kinases, adhesion molecules, the actin network and ion channels have been put forward as possible synaptic tags. Similarly, the local translation and degradation of proteins have also been considered as possible mechanisms to tag active synapses. As the evidence in support of all of these possibilities is good, it is probable that neurons do not use a single synaptic tag to mark synapses, but a wide variety of them.
-
The identity of the transcriptional products that interact with the tag remains unknown. Although a couple of candidates has been put forward, their precise identification constitutes one of the main challenges for this field.
Abstract
A synaptic tag transiently marks a synapse after activation in a way that allows the local recognition of transcriptional products to effect an enduring change in transmission efficiency. The idea of the synaptic tag as a single molecule might be misguided; instead, a process such as activation of local translation or cytoskeletal reorganization could mark a synapse. A change of this nature might be modulated directly or indirectly by transcriptional products that, for example, modulate translational activity, mRNA stability or protein degradation, or are ensconced in a cytoskeletal configuration, and thereby lead to long-term changes in synaptic strength.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Martin, K. C. et al. Synapse-specific, long-term facilitation of Aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell 91, 927–938 (1997).Shows that long-lasting, transcription-dependent forms of plasticity can occur in a synapse-specific manner; together with reference 12 , this paper provides evidence for synaptic tagging in Aplysia.
Frey, U. & Morris, R. G. Synaptic tagging and long-term potentiation. Nature 385, 533–536 (1997).This paper and reference 3 provide evidence for synaptic tagging in rat hippocampal neurons.
Frey, U. & Morris, R. G. Weak before strong: dissociating synaptic tagging and plasticity-factor accounts of late-LTP. Neuropharmacology 37, 545–552 (1998).
Shi, S. H. et al. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science 284, 1811–1816 (1999).
Tovar, K. R. & Westbrook, G. L. Mobile NMDA receptors at hippocampal synapses. Neuron 34, 255–264 (2002).
Ango, F. et al. Homer-dependent cell surface expression of metabotropic glutamate receptor type 5 in neurons. Mol. Cell. Neurosci. 20, 323–329 (2002).
El-Husseini, A. E. et al. Synaptic strength regulated by palmitate cycling on PSD-95. Cell 108, 849–863 (2002).
Lisman, J., Schulman, H. & Cline, H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nature Rev. Neurosci. 3, 175–190 (2002).
Osten, P., Valsamis, L., Harris, A. & Sacktor, T. C. Protein synthesis-dependent formation of protein kinase Mζ in long-term potentiation. J. Neurosci. 16, 2444–2451 (1996).
Ling, D. S. et al. Protein kinase Mζ is necessary and sufficient for LTP maintenance. Nature Neurosci. 5, 295–296 (2002).
Drier, E. A. et al. Memory enhancement and formation by atypical PKM activity in Drosophila melanogaster. Nature Neurosci. 5, 316–324 (2002).
Casadio, A. et al. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell 99, 221–237 (1999).
Chain, D. G., Hegde, A. N., Yamamoto, N., Liu-Marsh, B. & Schwartz, J. H. Persistent activation of cAMP-dependent protein kinase by regulated proteolysis suggests a neuron-specific function of the ubiquitin system in Aplysia. J. Neurosci. 15, 7592–7603 (1995).
Hegde, A. N. et al. Ubiquitin C-terminal hydrolase is an immediate–early gene essential for long-term facilitation in Aplysia. Cell 89, 115–126 (1997).
Chain, D. G. et al. Mechanisms for generating the autonomous cAMP-dependent protein kinase required for long-term facilitation in Aplysia. Neuron 22, 147–156 (1999).
Bailey, C. H. & Kandel, E. R. Structural changes accompanying memory storage. Annu. Rev. Physiol. 55, 397–426 (1993).
Schuster, C. M., Davis, G. W., Fetter, R. D. & Goodman, C. S. Genetic dissection of structural and functional components of synaptic plasticity. II. Fasciclin II controls presynaptic structural plasticity. Neuron 17, 655–667 (1996).
Davis, G. W., Schuster, C. M. & Goodman, C. S. Genetic dissection of structural and functional components of synaptic plasticity. III. CREB is necessary for presynaptic functional plasticity. Neuron 17, 669–679 (1996).
Bailey, C. H., Chen, M., Keller, F. & Kandel, E. R. Serotonin-mediated endocytosis of apCAM: an early step of learning-related synaptic growth in Aplysia. Science 256, 645–649 (1992).
Mayford, M., Barzilai, A., Keller, F., Schacher, S. & Kandel, E. R. Modulation of an NCAM-related adhesion molecule with long-term synaptic plasticity in Aplysia. Science 256, 638–644 (1992).
Dityatev, A., Dityateva, G. & Schachner, M. Synaptic strength as a function of post versus presynaptic expression of the neural cell adhesion molecule NCAM. Neuron 26, 207–117 (2000).
Tanaka, H. et al. Molecular modification of N-cadherin in response to synaptic activity. Neuron 25, 93–107 (2000).
Bozdagi, O., Shan, W., Tanaka, H., Benson, D. L. & Huntley, G. W. Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites, and required for potentiation. Neuron 28, 245–259 (2000).
Murase, S., Mosser, E. & Schuman, E. M. Depolarization drives β-catenin into neuronal spines promoting changes in synaptic structure and function. Neuron 35, 91–105 (2002).
Lu, Q. et al. δ-Catenin, an adhesive junction-associated protein which promotes cell scattering. J. Cell Biol. 144, 519–532 (1999).
Izawa, I., Nishizawa, M., Ohtakara, K. & Inagaki, M. Densin-180 interacts with δ-catenin/neural plakophilin-related armadillo repeat protein at synapses. J. Biol. Chem. 277, 5345–5350 (2002).
Fischer, M., Kaech, S., Wagner, U., Brinkhaus, H. & Matus, A. Glutamate receptors regulate actin-based plasticity in dendritic spines. Nature Neurosci. 3, 887–894 (2000).
Star, E. N., Kwiatkowski, D. J. & Murthy, V. N. Rapid turnover of actin in dendritic spines and its regulation by activity. Nature Neurosci. 5, 239–246 (2002).
Engert, F. & Bonhoeffer, T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399, 66–70 (1999).
Maletic-Savatic, M., Malinow, R. & Svoboda, K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science 283, 1923–1927 (1999).
Toni, N., Buchs, P. A., Nikonenko, I., Bron, C. R. & Muller, D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature 402, 421–425 (1999).
Hatada, Y., Wu, F., Sun, Z. Y., Schacher, S. & Goldberg, D. J. Presynaptic morphological changes associated with long-term synaptic facilitation are triggered by actin polymerization at preexisting varicosities. J. Neurosci. 20, RC82 (2000).
Colicos, M. A., Collins, B. E., Sailor, M. J. & Goda, Y. Remodeling of synaptic actin induced by photoconductive stimulation. Cell 107, 605–616 (2001).
Beaumont, V., Zhong, N., Froemke, R. C., Ball, R. W. & Zucker, R. S. Temporal synaptic tagging by Ih activation and actin: involvement in long-term facilitation and cAMP-induced synaptic enhancement. Neuron 33, 601–613 (2002).Provides evidence that the activation of presynaptic I h channels can serve as a synaptic tag at the crayfish neuromuscular junction. This tag does not interact with products of transcription; rather, it allows the neuron to integrate synaptic stimulation over time.
Beaumont, V., Zhong, N., Fletcher, R., Froemke, R. C. & Zucker, R. S. Phosphorylation and local presynaptic protein synthesis in calcium- and calcineurin-dependent induction of crayfish long-term facilitation. Neuron 32, 489–501 (2001).
Ostroff, L. E., Fiala, J. C., Allwardt, B. & Harris, K. M. Polyribosomes redistribute from dendritic shafts into spines with enlarged synapses during LTP in developing rat hippocampal slices. Neuron (in the press).Shows that strong synaptic stimulation increases the percentage of spines containing polyribosome clusters in the rat hippocampus.
Krichevsky, A. M. & Kosik, K. S. Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron 32, 683–696 (2001).Provides evidence that RNA granules are storage sites for mRNAs in distal dendrites, and that depolarization releases mRNAs from the granules to polysomes, where they are actively translated.
Rook, M. S., Lu, M. & Kosik, K. S. CaMKIIα 3′ untranslated region-directed mRNA translocation in living neurons: visualization by GFP linkage. J. Neurosci. 20, 6385–6393 (2000).
Khan, A., Pepio, A. M. & Sossin, W. S. Serotonin activates S6 kinase in a rapamycin-sensitive manner in Aplysia synaptosomes. J. Neurosci. 21, 382–391 (2001).
Scheetz, A. J., Nairn, A. C. & Constantine-Paton, M. NMDA receptor-mediated control of protein synthesis at developing synapses. Nature Neurosci. 3, 211–216 (2000).
Huang, Y. S., Jung, M. Y., Sarkissian, M. & Richter, J. D. N-methyl-d-aspartate receptor signaling results in Aurora kinase-catalyzed CPEB phosphorylation and αCaMKII mRNA polyadenylation at synapses. EMBO J. 21, 2139–2148 (2002).
Pinkstaff, J. K., Chappell, S. A., Mauro, V. P., Edelman, G. M. & Krushel, L. A. Internal initiation of translation of five dendritically localized neuronal mRNAs. Proc. Natl Acad. Sci. USA 98, 2770–2775 (2001).
Lohof, A. M., Ip, N. Y. & Poo, M. M. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature 363, 350–353 (1993).
Levine, E. S., Dreyfus, C. F., Black, I. B. & Plummer, M. R. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc. Natl Acad. Sci. USA 92, 8074–8077 (1995).
Kang, H. & Schuman, E. M. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science 267, 1658–1662 (1995).
Kovalchuk, Y., Hanse, E., Kafitz, K. W. & Konnerth, A. Postsynaptic induction of BDNF-mediated long-term potentiation. Science 295, 1729–1734 (2002).
Patterson, S. L., Grover, L. M., Schwartzkroin, P. A. & Bothwell, M. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron 9, 1081–1088 (1992).
Korte, M. et al. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl Acad. Sci. USA 92, 8856–8860 (1995).
Tao, X., Finkbeiner, S., Arnold, D. B., Shaywitz, A. J. & Greenberg, M. E. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 20, 709–726 (1998).
Tongiorgi, E., Righi, M. & Cattaneo, A. Activity-dependent dendritic targeting of BDNF and TrkB mRNAs in hippocampal neurons. J. Neurosci. 17, 9492–9505 (1997).
Steward, O., Wallace, C. S., Lyford, G. L. & Worley, P. F. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron 21, 741–751 (1998).
Palacios, I. M. & Johnston, D. S. Getting the message across: the intracellular localization of mRNAs in higher eukaryotes. Annu. Rev. Cell Dev. Biol. 17, 569–614 (2001).
DiAntonio, A. et al. Ubiquitination-dependent mechanisms regulate synaptic growth and function. Nature 412, 449–452 (2001).
Campbell, D. S. & Holt, C. E. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron 32, 1013–1026 (2001).
Callen, E. J. & Boyd, T. L. Examination of a backchaining/counterconditioning process during the extinction of conditioned fear. Behav. Res. Ther. 28, 261–271 (1990).
Steward, O. & Schuman, E. M. Protein synthesis at synaptic sites on dendrites. Annu. Rev. Neurosci. 24, 299–325 (2001).
Ouyang, Y., Rosenstein, A., Kreiman, G., Schuman, E. M. & Kennedy, M. B. Tetanic stimulation leads to increased accumulation of Ca2+/calmodulin-dependent protein kinase II via dendritic protein synthesis in hippocampal neurons. J. Neurosci. 19, 7823–7833 (1999).
Aakalu, G., Smith, W. B., Nguyen, N., Jiang, C. & Schuman, E. M. Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron 30, 489–502 (2001).
Job, C. & Eberwine, J. Identification of sites for exponential translation in living dendrites. Proc. Natl Acad. Sci. USA 98, 13037–13042 (2001).
Spencer, G. E. et al. Synthesis and functional integration of a neurotransmitter receptor in isolated invertebrate axons. J. Neurobiol. 44, 72–81 (2000).
Ghirardi, M., Montarolo, P. G. & Kandel, E. R. A novel intermediate stage in the transition between short- and long-term facilitation in the sensory to motor neuron synapse of Aplysia. Neuron 14, 413–420 (1995).
Sutton, M. A. & Carew, T. J. Parallel molecular pathways mediate expression of distinct forms of intermediate-term facilitation at tail sensory-motor synapses in Aplysia. Neuron 26, 219–231 (2000).
Sutton, M. A., Ide, J., Masters, S. E. & Carew, T. J. Interaction between amount and pattern of training in the induction of intermediate- and long-term memory for sensitization in Aplysia. Learn. Mem. 9, 29–40 (2002).
Sherff, C. M. & Carew, T. J. Coincident induction of long-term facilitation in Aplysia: cooperativity between cell bodies and remote synapses. Science 285, 1911–1914 (1999).Shows that coincident, subthreshold application of serotonin to somata and processes of Aplysia neurons produces LTF that is dependent on local protein synthesis in the process.
Kang, H. & Schuman, E. M. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science 273, 1402–1406 (1996).
Huber, K. M., Kayser, M. S. & Bear, M. F. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science 288, 1254–1257 (2000).
Miller, S. et al. Disruption of dendritic translation of CaMKIIα impairs stabilization of synaptic plasticity and memory consolidation. Neuron (in the press).Describes a mouse in which the α-CaMKII mRNA is not dendritically localized, and shows a role for local protein synthesis in late-phase LTP and long-term forms of memory.
Lanahan, A. & Worley, P. Immediate–early genes and synaptic function. Neurobiol. Learn. Mem. 70, 37–43 (1998). | PubMed
Leil, T. A., Ossadtchi, A., Cortes, J. S., Leahy, R. M. & Smith, D. J. Finding new candidate genes for learning and memory. J. Neurosci. Res. 68, 127–137 (2002).
Dubnau, J. & Tully, T. Gene discovery in Drosophila: new insights for learning and memory. Annu. Rev. Neurosci. 21, 407–444 (1998).
Grosshans, H. & Slack, F. J. Micro-RNAs: small is plentiful. J. Cell Biol. 156, 17–21 (2002).
Ambros, V. MicroRNAs: tiny regulators with great potential. Cell 107, 823–826 (2001).
Barco, A., Alarcon, J. M. & Kandel, E. R. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell 108, 689–703 (2002).Describes a mouse in which expression of a constitutively active form of the transcription factor CREB leads to a reduced threshold for producing long-lasting LTP. The subthreshold stimuli might produce tags that can capture the products of transcription that are induced by CREB activation.
Acknowledgements
We would like to thank M. Barad, M. Mayford, R. Moccia, E. Schuman, W. Sossin and O. Steward for their insightful comments on the manuscript.
Author information
Authors and Affiliations
Related links
Related links
DATABASES
LocusLink
ubiquitin carboxy-terminal hydrolase
FURTHER INFORMATION
Encyclopedia of Life Sciences
Glossary
- SCHAFFER COLLATERALS
-
Axons of the CA3 pyramidal cells of the hippocampus that form synapses with the apical dendrites of CA1 neurons.
- HOMER
-
A protein of the postsynaptic density that can interact with metabotropic glutamate receptors, regulating their membrane insertion and their interaction with other postsynaptic proteins.
- UBIQUITIN
-
A molecule that is attached to lysine residues of other proteins, often as a tag for their rapid cellular degradation by the proteasome.
- PROTEASOME
-
A protein complex responsible for degrading intracellular proteins that have been tagged for destruction by the addition of ubiquitin.
- HYPOMORPH
-
A mutant that expresses less than the normal amount of a given gene product.
- ADHERENS JUNCTION
-
A cell–cell junction also known as zonula adherens, which is characterized by the intracellular insertion of microfilaments. If intermediate filaments are inserted in lieu of microfilaments, the resulting junction is referred to as a desmosome.
- PHOTOCONDUCTIVE STIMULATION
-
A method whereby light can be used to stimulate the activity of subpopulations of synapses in vitro. Cells are cultured on a silicon chip and light is applied to single neurons. As the conductivity of silicon changes in response to light, it is possible to pair local illumination of single neurons with subthreshold electrical stimulation of the whole culture. So, the stimulus intensity will reach threshold for activation solely in the illuminated cell.
- INTERNAL RIBOSOME ENTRY SITE
-
A sequence that is inserted between the coding regions of two proteins and allows efficient assembly of the ribosome complex in the middle of a transcript, leading to translation of the second protein.
- MULTIPHOTON MICROSCOPY
-
A form of microscopy in which a fluorochrome that would normally be excited by a single photon is stimulated quasi-simultaneously by several photons of lower energy. Under these conditions, fluorescence increases as a function of the square of the light intensity, and decreases approximately as the square of the distance from the focus. Because of this behaviour, only fluorochrome molecules near the plane of focus are excited, greatly reducing light scattering and photodamage of the sample.
- MICRORNAS
-
Tiny transcripts of about 22 nucleotides. The functions of these RNAs remain obscure, although they probably serve as antisense regulators of the translation of other RNAs.
Rights and permissions
About this article
Cite this article
Martin, K., Kosik, K. Synaptic tagging — who's it?. Nat Rev Neurosci 3, 813–820 (2002). https://doi.org/10.1038/nrn942
Issue Date:
DOI: https://doi.org/10.1038/nrn942
This article is cited by
-
Excitation–transcription coupling, neuronal gene expression and synaptic plasticity
Nature Reviews Neuroscience (2023)
-
Spatial-Memory Formation After Spaced Learning Involves ERKs1/2 Activation Through a Behavioral-Tagging Process
Scientific Reports (2020)
-
How the epigenome integrates information and reshapes the synapse
Nature Reviews Neuroscience (2019)
-
Molecular Mechanisms of Early and Late LTP
Neurochemical Research (2019)
-
Forebrain-specific, conditional silencing of Staufen2 alters synaptic plasticity, learning, and memory in rats
Genome Biology (2017)