Abstract
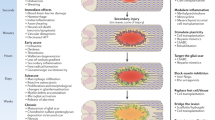
Several recent publications describe remarkably promising effects of transplanting olfactory ensheathing cells as a potential future method to repair human spinal cord injuries. But why were cells from the nose transplanted into the spinal cord? What are olfactory ensheathing cells, and how might they produce these beneficial effects? And more generally, what do we mean by spinal cord injury? To what extent can we compare repair in an animal to repair in a human?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Suzuki, M. & Raisman, G. The glial framework of central white matter tracts: segmented rows of contiguous interfascicular oligodendrocytes and solitary astrocytes give rise to a continuous meshwork of transverse and longitudinal processes in the adult rat fimbria. Glia 6, 222–235 (1992).
Chisholm, A. & Tessier-Lavigne, M. Conservation and divergence of axon guidance mechanisms. Curr. Opin. Neurobiol. 9, 603–615 (1999).
Li, Y., Field, P. M. & Raisman, G. Death of oligodendrocytes and microglial phagocytosis of myelin precede immigration of Schwann cells into the spinal cord. J. Neurocytol. 28, 417–427 (1999).
Crowe, M. J., Bresnahan, J. C., Shuman, S. L., Masters, J. N. & Beattie, M. S. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nature Med. 3, 73–76 (1997).
Davies, S. J. A. et al. Regeneration of adult axons in white matter tracts of the central nervous system. Nature 390, 680–683 (1997).
Fitch, M. T. & Silver, J. Activated macrophages and the blood–brain barrier: inflammation after CNS injury leads to increases in putative inhibitory molecules. Exp. Neurol. 148, 587–603 (1997).
Fawcett, J. W. Astrocytic and neuronal factors affecting axon regeneration in the damaged central nervous system. Cell Tissue Res. 290, 371–377 (1997).
Ramón y Cajal, S. Degeneration and Regeneration of the Nervous System (Hafner, New York, 1928).
Richardson, P. M., Issa, V. M. K. & Aguayo, A. J. Regeneration of long spinal axons in the rat. J. Neurocytol. 13, 165–182 (1984).
Trigueros, M. A., Sauvé, Y., Lund, R. D. & Vidal-Sanz, M. Selective innervation of retinorecipient brainstem nuclei by retinal ganglion cell axons regenerating through peripheral nerve grafts in adult rats. J. Neurosci. 20, 361–374 (2000).
Li, Y. & Raisman, G. Schwann cells induce sprouting in motor and sensory axons in the adult rat spinal cord. J. Neurosci. 14, 4050–4063 (1994).
Xu, X. M., Chen, A., Guénard, V., Kleitman, N. & Bunge, M. B. Bridging Schwann cell transplants promote axonal regeneration from both the rostral and caudal stumps of transected adult rat spinal cord. J. Neurocytol. 26, 1–16 (1997).
Paíno, C. L., Fernandez-Valle, C., Bates, M. L. & Bunge, M. B. Regrowth of axons in lesioned adult rat spinal cord: promotion by implants of cultured Schwann cells. J. Neurocytol. 23, 433–452 (1994).
Graziadei, P. P. C. & Montigraziadei, G. A. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J. Neurocytol. 8, 1–18 (1979).
Farbman, A. I. Olfactory neurogenesis: genetic or environmental controls? Trends Neurosci. 13, 362–365 (1990).
Graziadei, P. P. C., Levine, R. R. & Montigraziadei, G. A. Plasticity of connections of the olfactory sensory neuron: regeneration into the forebrain following bulbectomy in the neonatal mouse. Neuroscience 4, 713–727 (1979).
Ramón-Cueto, A. & Valverde, F. Olfactory bulb ensheathing glia: A unique cell type with axonal growth-promoting properties. Glia 14, 163–173 (1995).
Doucette, J. R. The glial cells in the nerve fiber layer of the rat olfactory bulb. Anat. Rec. 210, 385–391 (1984).
Raisman, G. Specialized neuroglial arrangement may explain the capacity of vomeronasal axons to reinnervate central neurons. Neuroscience 14, 237–254 (1985).
Devon, R. & Doucette, R. Olfactory ensheathing cells myelinate dorsal root ganglion neurites. Brain Res. 589, 175–179 (1992).
Barnett, S. C., Hutchins, A.-M. & Noble, M. Purification of olfactory nerve ensheathing cells from the olfactory bulb. Dev. Biol. 155, 337–350 (1993).
Ramón-Cueto, A. & Nieto-Sampedro, M. Glial cells from adult rat olfactory bulb: immunocytochemical properties of pure cultures of ensheathing cells. Neuroscience 47, 213–220 (1992).
Ramón-Cueto, A. & Nieto-Sampedro, M. Regeneration into the spinal cord of transected dorsal root axons is promoted by ensheathing glia transplants. Exp. Neurol. 127, 232–244 (1994).
Ramón-Cueto, A., Plant, G. W., Avila, J. & Bunge, M. B. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J. Neurosci. 18, 3803–3815 (1998).
Li, Y., Field, P. M. & Raisman, G. Regeneration of adult rat corticospinal axons induced by transplanted olfactory ensheathing cells. J. Neurosci. 18, 10514–10524 (1998).
Li, Y., Field, P. M. & Raisman, G. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science 277, 2000–2002. (1997).
Imaizumi, T., Langford, K. L., Waxman, S. G., Greer, C. A. & Kocsis, J. D. Transplanted olfactory ensheathing cells remyelinate and enhance axonal conduction in the demyelinated dorsal columns of the rat spinal cord. J. Neurosci. 18, 6176–6185 (1998).
Franklin, R. J.M., Gilson, J. M., Franceschini, I. A. & Barnett, S. C. Schwann cell-like myelination following transplantation of an olfactory bulb-ensheathing cell line into areas of demyelination in the adult CNS. Glia 17, 217–224 (1996).
Ramón-Cueto, A., Cordero, M. I., Santos-Benito, F. F. & Avila, J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron 25, 425–435 (2000).
Franklin, R. J.M. & Barnett, S. C. Olfactory ensheathing cells and CNS regeneration: The sweet smell of success? Neuron 28, 15–18 (2000).
Franceschini, I. A. & Barnett, S. C. Low-affinity NGF-receptor and E-N-CAM expression define two types of olfactory nerve ensheathing cells that share a common lineage. Dev. Biol. 173, 327–343 (1996).
Pixley, S. K. The olfactory nerve contains two populations of glia, identified both in vivo and in vitro. Glia 5, 269–284 (1992).
Ramón-Cueto, A. & Avila, J. Olfactory ensheathing glia: properties and function. Brain Res. Bull. 46, 175–187 (1998).
Barnett, S. C. et al. Identification of a human olfactory ensheathing cell that can effect transplant-mediated remyelination of demyelinated CNS axons. Brain 123, 1581–1588 (2000).
Weiss, S. et al. Is there a neural stem cell in the mammalian forebrain? Trends Neurosci. 19, 387–393 (1996).
Pagano, S. F. et al. Isolation and characterization of neural stem cells from the adult human olfactory bulb. Stem Cells 18, 295–300 (2000).
Olby, N. J. & Blakemore, W. F. Primary demyelination and regeneration of ascending axons in the dorsal funiculus of the rat spinal cord following photochemically induced injury. J. Neurocytol. 25, 465–480 (1996).
Verdú, E., Rodríguez, F. J., Gudiño-Cabrera, G., Nieto-Sampedro, M. & Navarro, X. Expansion of adult Schwann cells from mouse predegenerated peripheral nerves. J. Neurosci. Methods 99, 111–117 (2000).
Morrissey, T. K., Kleitman, N. & Bunge, R. P. Isolation and functional characterization of Schwann cells derived from adult peripheral nerve. J. Neurosci. 11, 2433–2442 (1991).
Casella, C. T. B., Bunge, R. P. & Wood, P. M. Improved method for harvesting human Schwann cells from mature peripheral nerve and expansion in vitro. Glia 17, 327–338 (1996).
Franklin, R. J. & Barnett, S. C. Do olfactory glia have advantages over Schwann cells for CNS repair? J. Neurosci. Res. 50, 665–672 (1997).
De Carlos, J. A., López-Mascaraque, L. & Valverde, F. Early olfactory fiber projections and cell migration into the rat telencephalon. Int. J. Dev. Neurosci. 14, 853–866 (1996).
Goldstein, B. J., Fang, H. S., Youngentob, S. L. & Schwob, J. E. Transplantation of multipotent progenitors from the adult olfactory epithelium. Neuroreport 9, 1611–1617 (1998).
Chuah, M. I., Tennent, R. & Jacobs, I. Response of olfactory Schwann cells to intranasal zinc sulfate irrigation. J. Neurosci. Res. 42, 470–478 (1995).
Liu, K. L., Chuah, M. I. & Lee, K. K. H. Soluble factors from the olfactory bulb attract olfactory Schwann cells. J. Neurosci. 15, 990–1000 (1995).
Kato, T., Honmou, O., Uede, T., Hashi, K. & Kocsis, J. D. Transplantation of human olfactory ensheathing cells elicits remyelination of demyelinated rat spinal cord. Glia 30, 209–218 (2000).
Merline, M. & Kalil, K. Cell death of corticospinal neurons is induced by axotomy before but not after innervation of spinal targets. J. Comp. Neurol. 296, 506–516 (1990).
Tseng, G. F. & Prince, D. A. Structural and functional alterations in rat corticospinal neurons after axotomy. J. Neurophysiol. 75, 248–267 (1996).
Fishman, P. S. & Kelly, J. P. The fate of severed corticospinal axons. Neurology 34, 1161–1167 (1984).
Li, Y. & Raisman, G. Sprouts from cut corticospinal axons persist in the presence of astrocytic scarring in long-term lesions of the adult rat spinal cord. Exp. Neurol. 134, 102–111 (1995).
Kuang, R. Z. & Kalil, K. Specificity of corticospinal axon arbors sprouting into denervated contralateral spinal cord. J. Comp. Neurol. 302, 461–472 (1990).
Weidner, N., Ner, A., Salimi, N. & Tuszynski, M. H. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc. Natl Acad. Sci. USA 98, 3513–3518 (2001).
Li, W. W. Y., Yew, D. T. W., Chuah, M. I., Leung, P. C. & Tsang, D. S. C. Axonal sprouting in the hemisected adult rat spinal cord. Neuroscience 61, 133–139 (1994).
Aoki, M., Fujito, Y., Satomi, H., Kurosawa, Y. & Kasaba, T. The possible role of collateral sprouting in the functional restoration of corticospinal connections after spinal hemisection. Neurosci. Res. 3, 617–627 (1986).
Kapfhammer, J. P. Axon sprouting in the spinal cord: growth promoting and growth inhibitory mechanisms. Anat. Embryol. (Berl.) 196, 417–426 (1997).
Eidelberg, E. Consequences of spinal cord lesions upon motor function, with special reference to locomotor activity. Prog. Neurobiol. 17, 185–202 (1981).
Lawrence, D. G. & Kuypers, H. G. J. M. The functional organization of the motor system. I. The effects of bilateral pyramidal lesions. Brain 91, 1–14 (1968).
Heffner, R. S. & Masterton, R. B. The role of the corticospinal tract in the evolution of human digital dexterity. Brain Behav. Evol. 23, 165–183 (1983).
Bregman, B. S. et al. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Neuron 15, 498–501 (1995).
Kuypers, H. G. J. M. Progress in Brain Research Vol. 11 (eds Eccles, J. C. & Schadé, J. P.) 178–202 (Elsevier, Amsterdam, 1964).
Galea, M. P. & Darian-Smith, I. Multiple corticospinal neuron populations in the macaque monkey are specified by their unique cortical origins, spinal terminations, and connections. Cereb. Cortex 4, 166–194 (1994).
Rossignol, S. & Dubuc, R. Spinal pattern generation. Curr. Opin. Neurobiol. 4, 894–902 (1994).
Hultborn, H. et al. How do we approach the locomotor network in the mammalian spinal cord? Ann. NY Acad. Sci. 860, 70–82 (1998).
Wickelgren, I. Teaching the spinal cord to walk. Science 279, 319–321 (1998).
Harkema, S. J. et al. Human lumbosacral spinal cord interprets loading during stepping. J. Neurophysiol. 77, 797–811 (1997).
Rossignol, S. Locomotion and its recovery after spinal injury. Curr. Opin. Neurobiol. 10, 708–716 (2000).
Edgerton, V. R. et al. Use-dependent plasticity in spinal stepping and standing. Adv. Neurol. 72, 233–247 (1997).
Wernig, A., Müller, S., Nanassy, A. & Cagol, E. Laufband therapy based on 'rules of spinal locomotion' is effective in spinal cord injured persons. Eur. J. Neurosci. 7, 823–829 (1995).
Castro, A. J. The effects of cortical ablations on digital usage in the rat. Brain Res. 37, 173–185 (1972).
Castro, A. J. Motor performance in rats. The effects of pyramidal tract section. Brain Res. 44, 313–323 (1972).
Xu, X. M., Zhang, S. X., Li, H. Y., Aebischer, P. & Bunge, M. B. Regrowth of axons into the distal spinal cord through a Schwann-cell-seeded mini-channel implanted into hemisected adult rat spinal cord. Eur. J. Neurosci. 11, 1723–1740 (1999).
Joosten, E. A. J., Bär, P. R. & Gispen, W. H. Collagen implants and cortico-spinal axonal growth after mid- thoracic spinal cord lesion in the adult rat. J. Neurosci. Res. 41, 481–490 (1995).
Bomze, H. M., Bulsara, K. R., Iskandar, B. J., Caroni, P. & Pate Skene, J. H. Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nature Neurosci. 4, 38–43 (2001).
McKerracher, L. et al. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron 13, 805–811 (1994).
Bregman, B. S. Regeneration in the spinal cord. Curr. Opin. Neurobiol. 8, 800–807 (1998).
Olson, L. Regeneration in the adult central nervous system: experimental repair strategies. Nature Med. 3, 1329–1335 (1997).
Lehmann, M. et al. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J. Neurosci. 19, 7537–7547 (1999).
Cai, D., Shen, Y., De Bellard, M., Tang, S. & Filbin, M. T. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron 22, 89–101 (1999).
Valverde, F. & Lopez-Mascaraque, L. Neuroglial arrangements in the olfactory glomeruli of the hedgehog. J. Comp. Neurol. 307, 658–674 (1991).
Ramón y Cajal, S. R. Histologie du Système Nerveux de l'Homme et des Vertébrés (Maloine, Paris, 1911).
Acknowledgements
Supported by the Medical Research Council the British Neurological Research Trust and the International Spinal Research Trust.
Author information
Authors and Affiliations
Related links
Related links
ENCYCLOPEDIA OF LIFE SCIENCES
Traumatic central nervous system injury
Rights and permissions
About this article
Cite this article
Raisman, G. Olfactory ensheathing cells — another miracle cure for spinal cord injury?. Nat Rev Neurosci 2, 369–375 (2001). https://doi.org/10.1038/35072576
Issue Date:
DOI: https://doi.org/10.1038/35072576
This article is cited by
-
Lipid Rafts from Olfactory Ensheathing Cells: Molecular Composition and Possible Roles
Cellular and Molecular Neurobiology (2021)
-
Cell transplantation therapy for spinal cord injury
Nature Neuroscience (2017)
-
Cell migration in the developing rodent olfactory system
Cellular and Molecular Life Sciences (2016)
-
Phenotypic Modulation and Neuroprotective Effects of Olfactory Ensheathing Cells: a Promising Tool for Cell Therapy
Stem Cell Reviews and Reports (2016)
-
Immunocytochemical characterisation of ensheathing glia in the olfactory and vomeronasal systems of Ambystoma mexicanum (Caudata: Ambystomatidae)
Brain Structure and Function (2016)