Key Points
-
Observational and experimental studies dating back to the 1950s demonstrate that mammals spontaneously help distressed conspecifics. Research emphasizes the untrained, unrewarded nature of this behaviour, which is also biased towards familiar individuals, thus arguing against explanations that are exclusively based on associative learning or conditioning.
-
The perception–action model extends an existing motor theory on overlapping representations to emotional phenomena; it states that observers who attend to a target's state understand and 'feel into' it through personal distributed representations of the target, the state and the situation. Easily observed manifestations of this mechanism are emotional contagion and motor mimicry, which have been demonstrated in many animals. In cognitive forms of empathy, the same representations are accessed from the top-down.
-
Experiments on two common mammalian expressions of empathy — the consolation of distressed individuals and spontaneous assistance to those in need — support the crucial role of caught distress and arousal because these behaviours are suppressed by anti-anxiety medication and engage the same neuropeptide system that supports social attachment.
-
The Russian-doll model seeks to arrange forms of empathy into layers that are built on top of each other — with the layers ranging from emotional contagion to more cognitive forms of empathy — in a functionally integrated whole based on perception–action processes. Perspective-taking is well developed in some non-human species, as manifested by theory-of-mind and targeted helping.
-
One can segregate emotional and cognitive empathy (as well as felt and observed states) in the brains of observers, but all forms require some initial access to the observer's distributed, shared, personal representations of the target's state. At least in the initial phase of processing, this access helps to decode the target's state and provide subsequent processing with content and meaning, even if the shared state is not experienced, or is incomplete or inaccurate.
-
Empathic pain does not usually include the peripheral sensation of the target's injury, but it can include sensory information when the stimuli and task instructions emphasize the specific nature of the feeling at the location of the injury.
Abstract
Recent research on empathy in humans and other mammals seeks to dissociate emotional and cognitive empathy. These forms, however, remain interconnected in evolution, across species and at the level of neural mechanisms. New data have facilitated the development of empathy models such as the perception–action model (PAM) and mirror-neuron theories. According to the PAM, the emotional states of others are understood through personal, embodied representations that allow empathy and accuracy to increase based on the observer's past experiences. In this Review, we discuss the latest evidence from studies carried out across a wide range of species, including studies on yawn contagion, consolation, aid-giving and contagious physiological affect, and we summarize neuroscientific data on representations related to another's state.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Preston, S. D. & de Waal, F. B. M. Empathy: its ultimate and proximate bases. Behav. Brain Sci. 25, 1–71 (2002). This paper offers the first summary of empathy as a perception–action process and reviews evidence from multiple levels of analysis.
Goldman, A. I. Simulating Minds: the Philosophy, Psychology, and Neuroscience of Mindreading (Oxford Univ. Press, 2006).
Baron-Cohen, S. in The Lost Self: Pathologies of the Brain and Identity (eds Feinberg, T. E. & Keenan, J. P.) 166–180 (Oxford Univ. Press, 2005).
Zahavi, D. Simulation, projection and empathy. Conscious. Cogn. 17, 514–522 (2008).
Lipps, T. Einfühlung, innere nachahmung und organenempfindungen [German]. Arch. Gesamte Psychol. 1, 465–519 (1903).
de Waal, F. B. M. Putting the altruism back into altruism: the evolution of empathy. Annu. Rev. Psychol. 59, 279–300 (2008).
Preston, S. D. & Hofelich, A. J. The many faces of empathy: parsing empathic phenomena through a proximate, dynamic-systems view of representing the other in the self. Emot. Rev. 4, 24–33 (2012).
Zahn-Waxler, C. & Radke-Yarrow, M. The origins of empathic concern. Motiv. Emot. 14, 107–130 (1990).
Walter, H. Social cognitive neuroscience of empathy: concepts, circuits, and genes. Emot. Rev. 4, 9–17 (2012).
Darwin, C. The Descent of Man, and Selection in Relation to Sex (Princeton Univ. Press, 1982).
Plutchik, R. in Empathy and its Development (eds Eisenberg, N. & Strayer, J.) 3–46 (Cambridge Univ. Press, 1987).
de Waal, F. B. M. Good Natured (Harvard Univ. Press, 1996).
Krupenye, C., Kano, F., Hirata, S., Call, J. & Tomasello, M. Great apes anticipate that other individuals will act according to false beliefs. Science 354, 110–114 (2016).
de Waal, F. B. M. The Age of Empathy (Harmony Books, 2009).
Yamamoto, S., Humle, T. & Tanaka, M. Chimpanzees' flexible targeted helping based on an understanding of conspecifics' goals. Proc. Natl Acad. Sci. USA 109, 3588–3592 (2012).
Hattori, Y., Leimgruber, K., Fujita, K. & de Waal, F. B. M. Food-related tolerance in capuchin monkeys (Cebus apella) varies with knowledge of the partner's previous food-consumption. Behaviour 149, 171–185 (2012).
Bateson, P. & Laland, K. N. Tinbergen's four questions: an appreciation and an update. Trends Ecol. Evol. 28, 712–718 (2013).
Tinbergen, N. On aims and methods of ethology [German]. Z. Tierpsychol. 20, 410–433 (1963).
Church, R. M. Emotional reactions of rats to the pain of others. J. Comp. Physiol. Psychol. 52, 132–134 (1959). This is the first rodent study to analyse emotional responses to the distress of others.
Rice, G. E. & Gainer, P. “Altruism” in the albino rat. J. Comp. Physiol. Psychol. 55, 123–125 (1962).
Masserman, J. H., Wechkin, S. & Terris, W. “Altruistic” behavior in rhesus monkeys. Am. J. Psychiatry 121, 584–585 (1964).
Wechkin, S., Masserman, J. H. & Terris, W. Shock to a conspecific as an aversive stimulus. Psychon. Sci. 1, 47–48 (1964).
Miller, R. E., Murphy, J. V. & Mirsky, I. A. Relevance of facial expression and posture as cues in communication of affect between monkeys. AMA Arch. Gen. Psychiatry 1, 480–488 (1959).
Yerkes, R. M. Almost Human (Century, 1925).
Ladygina-Kohts, N. N. Infant Chimpanzee and Human Child (ed. de Waal, F. B. M.) (Oxford Univ. Press, 2001)
de Waal, F. B. M. & van Roosmalen, A. Reconciliation and consolation among chimpanzees. Behav. Ecol. Sociobiol. 5, 55–66 (1979). This is the first non-human animal study to define and analyse consolation behaviour.
Zahn-Waxler, C., Radke-Yarrow, M., Wagner, E. & Chapman, M. Development of concern for others. Dev. Psychol. 28, 126–136 (1992). This is a pioneering study of the early development of human consolation behaviour.
Liddle, M. J. E., Bradley, B. S. & Mcgrath, A. Baby empathy: infant distress and peer prosocial responses. Infant Ment. Health J. 36, 446–458 (2015).
Roth-Hanania, R., Davidov, M. & Zahn-Waxler, C. Empathy development from 8 to 16 months: early signs of concern for others. Infant Behav. Dev. 34, 447–458 (2011).
Dimberg, U., Thunberg, M. & Elmehed, K. Unconscious facial reactions to emotional facial expressions. Psychol. Sci. 11, 86–89 (2000). This study is part of a series of studies on facial mimicry that first demonstrated the spontaneous nature of human empathic reactions.
Mancini, G., Ferrari, P. F. & Palagi, E. Rapid facial mimicry in geladas. Sci. Rep. 3, 1527 (2013).
Ross, M. D., Menzler, S. & Zimmermann, E. Rapid facial mimicry in orangutan play. Biol. Lett. 4, 27–30 (2008).
Dimberg, U., Andréasson, P. & Thunberg, M. Emotional empathy and facial reactions to facial expressions. J. Psychophysiol. 25, 26–31 (2011).
Campbell, M. W. & de Waal, F. B. M. Ingroup-outgroup bias in contagious yawning by chimpanzees supports link to empathy. PLoS ONE 6, e18283 (2011).
Norscia, I. & Palagi, E. Yawn contagion and empathy in Homo sapiens. PLoS ONE 6, e28472 (2011).
Romero, T., Konno, A. & Hasegawa, T. Familiarity bias and physiological responses in contagious yawning by dogs support link to empathy. PLoS ONE 8, e71365 (2013).
Gallup, A. C., Swartwood, L., Militello, J. & Sackett, S. Experimental evidence of contagious yawning in budgerigars (Melopsittacus undulatus). Anim. Cogn. 18, 1051–1058 (2015).
Silva, K., Bessa, J. & de Sousa, L. Auditory contagious yawning in domestic dogs (Canis familiaris): first evidence for social modulation. Anim. Cogn. 15, 721–724 (2012).
Visalberghi, E. & Fragaszy, D. M. in 'Language' and Intelligence in Monkeys and Apes: Comparative Developmental Perspectives (eds Parker, S. T. & Gibson, K. R.) 247–273 (Cambridge Univ. Press, 1990).
Ferrari, P. F. et al. Neonatal imitation in rhesus macaques. PLoS Biol. 4, e302 (2006).
Paukner, A., Suomi, S. J., Visalberghi, E. & Ferrari, P. F. Capuchin monkeys display affiliation toward humans who imitate them. Science 325, 880–883 (2009).
Perry, S. et al. Social conventions in wild white-faced capuchin monkeys: evidence for traditions in a neotropical primate. Curr. Anthropol. 44, 241–268 (2003).
Dindo, M., Whiten, A. & de Waal, F. B. M. In-group conformity sustains different foraging traditions in capuchin monkeys (Cebus apella). PLoS ONE 4, e7858 (2009).
Horner, V. & Whiten, A. Causal knowledge and imitation/emulation switching in chimpanzees (Pan troglodytes) and children (Homo sapiens). Anim. Cogn. 8, 164–181 (2005).
Fuhrmann, D., Ravignani, A., Marshall-Pescini, S. & Whiten, A. Synchrony and motor mimicking in chimpanzee observational learning. Sci. Rep. 4, 5283 (2014).
Hopper, L. M., Lambeth, S. P., Schapiro, S. J. & Whiten, A. Observational learning in chimpanzees and children studied through 'ghost' conditions. Proc. R. Soc. B Biol. Sci. 275, 835–840 (2008).
Langford, D. J. et al. Social modulation of pain as evidence for empathy in mice. Science 312, 1967–1970 (2006). This paper provides compelling evidence of emotional contagion in mice and set the stage for subsequent rodent research.
Langford, D. J. et al. Social approach to pain in laboratory mice. Soc. Neurosci. 5, 163–170 (2010).
Martin, L. J. et al. Reducing social stress elicits emotional contagion of pain in mouse and human strangers. Curr. Biol. 25, 326–332 (2015).
Burkett, J. et al. Oxytocin-dependent consolation behavior in rodents. Science 351, 375–378 (2016).
Buchanan, T. W., Bagley, S. L., Stansfield, R. B. & Preston, S. D. The empathic, physiological resonance of stress. Soc. Neurosci. 7, 191–201 (2012). This paper provides the first demonstration that stress — at the physiological level — spreads from one stressed human participant to empathic observers.
Buchanan, T. W. & Preston, S. D. Stress leads to prosocial action in immediate need situations. Front. Behav. Neurosci. http://dx.doi.org/10.3389/fnbeh.2014.00005 (2014).
Clay, Z. & de Waal, F. B. M. Development of socio-emotional competence in bonobos. Proc. Natl Acad. Sci. USA 110, 18121–18126 (2013).
Fries, A. B. W. & Pollak, S. D. Emotion understanding in postinstitutionalized Eastern European children. Dev. Psychopathol. 16, 355–369 (2004).
Kagan, J. Human morality is distinctive. J. Conscious. Stud. 7, 46–48 (2000).
Fehr, E. & Fischbacher, U. The nature of human altruism. Nature 425, 785–791 (2003).
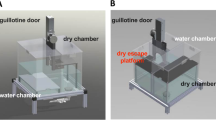
Bartal, I. B.-A., Decety, J. & Mason, P. Empathy and pro-social behavior in rats. Science 334, 1427–1430 (2011).
Sato, N., Tan, L., Tate, K. & Okada, M. Rats demonstrate helping behavior toward a soaked conspecific. Anim. Cogn. 18, 1039–1047 (2015).
Romero, T., Castellanos, M. A. & de Waal, F. B. M. Consolation as possible expression of sympathetic concern among chimpanzees. Proc. Natl Acad. Sci. USA 107, 12110–12115 (2010).
Clay, Z. & de Waal, F. B. M. Bonobos respond to distress in others: consolation across the age spectrum. PLoS ONE 8, e55206 (2013).
Palagi, E., Dall'Olio, S., Demuru, E. & Stanyon, R. Exploring the evolutionary foundations of empathy: consolation in monkeys. Evol. Hum. Behav. 35, 341–349 (2014).
Cools, A. K., Van Hout, A. J. M. & Nelissen, M. H. Canine reconciliation and third-party-initiated postconflict affiliation: do peacemaking social mechanisms in dogs rival those of higher primates? Ethology 114, 53–63 (2008).
Plotnik, J. M. & de Waal, F. B. M. Asian elephants (Elephas maximus) reassure others in distress. PeerJ 2, e278 (2014).
Seed, A. M., Clayton, N. S. & Emery, N. J. Postconflict third-party affiliation in rooks, Corvus frugilegus. Curr. Biol. 17, 152–158 (2007).
Fraser, O. N., Stahl, D. & Aureli, F. Stress reduction through consolation in chimpanzees. Proc. Natl Acad. Sci. USA 105, 8557–8562 (2008).
Aureli, F., Preston, S. D. & de Waal, F. B. M. Heart rate responses to social interactions in free-moving rhesus macaques (Macaca mulatta): a pilot study. J. Comp. Psychol. 113, 59–65 (1999).
Ben-Ami Bartal, I. et al. Anxiolytic treatment impairs helping behavior in rats. Front. Psychol. 7, 850 (2016).
Warneken, F., Hare, B., Melis, A. P., Hanus, D. & Tomasello, M. Spontaneous altruism by chimpanzees and young children. PLoS Biol. 5, e184 (2007).
Warneken, F. & Tomasello, M. Extrinsic rewards undermine altruistic tendencies in 20-month-olds. Motiv. Sci. 1, 43–48 (2014).
Andreoni, J. Giving with impure altruism: applications to charity and Ricardian equivalence. J. Polit. Econ. 97, 1447–1458 (1989).
Wager, T. D. et al. Pain in the ACC? Proc. Natl Acad. Sci. USA 113, E2474–E2475 (2016).
Ballesta, S. & Duhamel, J.-R. Rudimentary empathy in macaques' social decision-making. Proc. Natl Acad. Sci. USA 112, 15516–15521 (2015).
Christov-Moore, L. & Iacoboni, M. Self–other resonance, its control and prosocial inclinations: brain–behavior relationships. Hum. Brain Mapp. 37, 1544–1558 (2016).
Hoffman, M. L. Is altruism part of human nature? J. Pers. Soc. Psychol. 40, 121–137 (1981).
Nowbahari, E., Scohier, A., Durand, J.-L. & Hollis, K. L. Ants, Cataglyphis cursor, use precisely directed rescue behavior to free entrapped relatives. PLoS ONE 4, e6573 (2009).
Vasconcelos, M., Hollis, K., Nowbahari, E. & Kacelnik, A. Pro-sociality without empathy. Biol. Lett. 8, 910–912 (2012).
Wilson, E. O. A chemical releaser of alarm and digging behavior in the ant Pogonomyrmex badius (Latreille). Psyche 65, 41–51 (1958).
Preston, S. D. The origins of altruism in offspring care. Psychol. Bull. 139, 1305–1341 (2013). This is a comprehensive review of the offspring-care perspective on how altruism and heroic rescue evolved and are encoded in the brain.
MacLean, P. D. Brain evolution relating to family, play, and the separation call. Arch. Gen. Psychiatry 42, 405–417 (1985).
Sivaselvachandran, S., Acland, E. L., Abdallah, S. & Martin, L. J. Behavioral and mechanistic insight into rodent empathy. Neurosci. Biobehav. Rev. http://dx.doi.org/10.1016/j.neubiorev.2016.06.007 (2016).
Edgar, J., Lowe, J., Paul, E. & Nicol, C. Avian maternal response to chick distress. Proc. R. Soc. B Biol. Sci. 278, 3129–3134 (2011).
Doody, J. S., Burghardt, G. M. & Dinets, V. Breaking the social–non-social dichotomy: a role for reptiles in vertebrate social behavior research? Ethology 119, 95–103 (2013).
Donaldson, Z. R. & Young, L. J. Oxytocin, vasopressin, and the neurogenetics of sociality. Science 322, 900–904 (2008).
Chen, Q., Panksepp, J. B. & Lahvis, G. P. Empathy is moderated by genetic background in mice. PLoS ONE 4, e4387 (2009).
Dittus, W. P. & Ratnayeke, S. M. Individual and social behavioral responses to injury in wild toque macaques (Macaca sinica). Int. J. Primatol. 10, 215–234 (1989).
De Vignemont, F. & Singer, T. The empathic brain: how, when and why? Trends Cogn. Sci. 10, 435–441 (2006).
de Waal, F. B. M. Chimpanzee Politics (John's Hopkins Univ. Press, 1998).
Wilson, M. L. et al. Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature 513, 414–417 (2014).
Lanzetta, J. T. & Englis, B. G. Expectations of cooperation and competition and their effects on observers' vicarious emotional responses. J. Pers. Soc. Psychol. 56, 543–554 (1989).
Prinz, W. Perception and action planning. Eur. J. Cogn. Psychol. 9, 129–154 (1997).
di Pellegrino, G., Fadiga, L., Fogassi, L., Gallese, V. & Rizzolatti, G. Understanding motor events: a neurophysiological study. Exp. Brain Res. 91, 176–180 (1992). This study reports the discovery of mirror neurons, which are a subclass of cells that activate similarly when a macaque or the experimenter performs a goal-directed motor action.
Grafton, S. T., Fadiga, L., Arbib, M. A. & Rizzolatti, G. Premotor cortex activation during observation and naming of familiar tools. Neuroimage 6, 231–236 (1997).
Adolphs, R., Damasio, H., Tranel, D., Cooper, G. & Damasio, A. R. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. J. Neurosci. 20, 2683–2690 (2000). This study offers early support for the PAM by showing that damage to the somatosensory cortex (where the body is represented) impairs an individual's ability to recognize the facial emotions of others.
Jackson, P. L., Brunet, E., Meltzoff, A. N. & Decety, J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia 44, 752–761 (2006).
Singer, T. & Lamm, C. The social neuroscience of empathy. Ann. NY Acad. Sci. 1156, 81–96 (2009).
Zaki, J. & Ochsner, K. N. The neuroscience of empathy: progress, pitfalls and promise. Nat. Neurosci. 15, 675–680 (2012).
Wicker, B. et al. Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron 40, 655–664 (2003).
Preston, S. D. et al. The neural substrates of cognitive empathy. Soc. Neurosci. 2, 254–275 (2007).
Morelli, S. A. & Lieberman, M. D. The role of automaticity and attention in neural processes underlying empathy for happiness, sadness, and anxiety. Front. Hum. Neurosci. 7, 160 (2013).
Zaki, J., Wager, T. D., Singer, T., Keysers, C. & Gazzola, V. The anatomy of suffering: understanding the relationship between nociceptive and empathic pain. Trends Cogn. Sci. 20, 249–259 (2016).
Mohr, A. H., Kross, E. & Preston, S. D. Devil in the details: effects of depression on the prosocial response depend on timing and similarity. Adapt. Hum. Behav. Physiol. 2, 281–297 (2016).
Hodges, S. D., Kiel, K. J., Kramer, A. D., Veach, D. & Villanueva, B. R. Giving birth to empathy: the effects of similar experience on empathic accuracy, empathic concern, and perceived empathy. Pers. Soc. Psychol. Bull. 36, 398–409 (2010).
Völlm, B. A. et al. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage 29, 90–98 (2006).
Lamm, C., Nusbaum, H. C., Meltzoff, A. N. & Decety, J. What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS ONE 2, e1292 (2007).
Nummenmaa, L., Hirvonen, J., Parkkola, R. & Hietanen, J. K. Is emotional contagion special? An fMRI study on neural systems for affective and cognitive empathy. Neuroimage 43, 571–580 (2008).
Schnell, K., Bluschke, S., Konradt, B. & Walter, H. Functional relations of empathy and mentalizing: an fMRI study on the neural basis of cognitive empathy. Neuroimage 54, 1743–1754 (2011).
Blair, R. J. R. Responding to the emotions of others: dissociating forms of empathy through the study of typical and psychiatric populations. Conscious. Cogn. 14, 698–718 (2005).
Derntl, B., Seidel, E.-M., Schneider, F. & Habel, U. How specific are emotional deficits? A comparison of empathic abilities in schizophrenia, bipolar and depressed patients. Schizophr. Res. 142, 58–64 (2012).
Samson, D., Apperly, I. A., Chiavarino, C. & Humphreys, G. W. Left temporoparietal junction is necessary for representing someone else's belief. Nat. Neurosci. 7, 499–500 (2004).
Saxe, R. & Wexler, A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia 43, 1391–1399 (2005).
Adolphs, R., Baron-Cohen, S. & Tranel, D. Impaired recognition of social emotions following amygdala damage. J. Cogn. Neurosci. 14, 1264–1274 (2002).
Blair, R. J. R. Fine cuts of empathy and the amygdala: dissociable deficits in psychopathy and autism. Q. J. Exp. Psychol. (Hove) 61, 157–170 (2008).
Marsh, A. A. Neural, cognitive, and evolutionary foundations of human altruism. Wiley Interdiscip. Rev. Cogn. Sci. 7, 59–71 (2016).
Singer, T. et al. Empathy for pain involves the affective but not sensory components of pain. Science 303, 1157–1162 (2004).
Morrison, I., Lloyd, D., di Pellegrino, G. & Roberts, N. Vicarious responses to pain in anterior cingulate cortex: is empathy a multisensory issue? Cogn. Affect. Behav. Neurosci. 4, 270–278 (2004).
Jackson, P. L., Meltzoff, A. N. & Decety, J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage 24, 771–779 (2005).
Morrison, I. & Downing, P. E. Organization of felt and seen pain responses in anterior cingulate cortex. Neuroimage 37, 642–651 (2007).
Lamm, C., Decety, J. & Singer, T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage 54, 2492–2502 (2011).
Mischkowski, D., Crocker, J. & Way, B. M. From painkiller to empathy killer: acetaminophen (paracetamol) reduces empathy for pain. Soc. Cogn. Affect. Neurosci. 11, 1345–1353 (2016).
Rütgen, M. et al. Placebo analgesia and its opioidergic regulation suggest that empathy for pain is grounded in self pain. Proc. Natl Acad. Sci. USA 112, E5638–E5646 (2015).
Rutgen, M., Seidel, E. M., Riecansky, I. & Lamm, C. Reduction of empathy for pain by placebo analgesia suggests functional equivalence of empathy and first-hand emotion experience. J. Neurosci. 35, 8938–8947 (2015).
Danziger, N., Prkachin, K. M. & Willer, J.-C. Is pain the price of empathy? The perception of others' pain in patients with congenital insensitivity to pain. Brain 129, 2494–2507 (2006).
Lieberman, M. D. & Eisenberger, N. I. The dorsal anterior cingulate cortex is selective for pain: results from large-scale reverse inference. Proc. Natl Acad. Sci. USA 112, 15250–15255 (2015).
Krishnan, A. et al. Somatic and vicarious pain are represented by dissociable multivariate brain patterns. eLife 5, e15166 (2016).
Fan, Y., Duncan, N. W., de Greck, M. & Northoff, G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci. Biobehav. Rev. 35, 903–911 (2011). This paper offers a meta-analysis of the role of the dACC–aMCC–SMA and the bilateral anterior insula across empathy tasks, demonstrating a domain-general role with similar recruitment for self and other experiences.
Corradi-Dell'Acqua, C., Tusche, A., Vuilleumier, P. & Singer, T. Cross-modal representations of first-hand and vicarious pain, disgust and fairness in insular and cingulate cortex. Nat. Commun. 7, 10904 (2016). In this study, the anterior insula and aMCC are engaged across pain, disgust and unfairness, showing that these regions are not pain specific but participate in various negative self and other experiences.
Danziger, N., Faillenot, I. & Peyron, R. Can we share a pain we never felt? Neural correlates of empathy in patients with congenital insensitivity to pain. Neuron 61, 203–212 (2008).
Craig, A. D. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 3, 655–666 (2002).
de la Vega, A., Chang, L. J., Banich, M. T., Wager, T. D. & Yarkoni, T. Large-scale meta-analysis of human medial frontal cortex reveals tripartite functional organization. J. Neurosci. 36, 6553–6562 (2016).
Shackman, A. J. et al. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 12, 154–167 (2011).
Morrison, I., Peelen, M. V. & Downing, P. E. The sight of others' pain modulates motor processing in human cingulate cortex. Cereb. Cortex 17, 2214–2222 (2007).
Carr, L., Iacoboni, M., Dubeau, M. C., Mazziotta, J. C. & Lenzi, G. L. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc. Natl Acad. Sci. USA 100, 5497–5502 (2003).
Calder, A. J., Keane, J., Manes, F., Antoun, N. & Young, A. W. Impaired recognition and experience of disgust following brain injury. Nat. Neurosci. 3, 1077–1078 (2000).
Bechara, A., Damasio, H. & Damasio, A. R. Emotion, decision making and the orbitofrontal cortex. Cereb. Cortex 10, 295–307 (2000). This paper shows how the orbitofrontal cortex informs decisions from one's own simulated affective state, allowing humans to imagine how another person feels and how they would feel as a result of possible decision outcomes.
Gallese, V., Keysers, C. & Rizzolatti, G. A unifying view of the basis of social cognition. Trends Cogn. Sci. 8, 396–403 (2004).
Iacoboni, M. Imitation, empathy, and mirror neurons. Annu. Rev. Psychol. 60, 653–670 (2008).
Mukamel, R., Ekstrom, A. D., Kaplan, J., Iacoboni, M. & Fried, I. Single-neuron responses in humans during execution and observation of actions. Curr. Biol. 20, 750–756 (2010).
Keysers, C. & Gazzola, V. Social neuroscience: mirror neurons recorded in humans. Curr. Biol. 20, R353–R354 (2010).
Shamay-Tsoory, S. G., Aharon-Peretz, J. & Perry, D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain 132, 617–627 (2009).
Acknowledgements
The authors thank their sources of funding during the preparation of this manuscript, including support for the Living Links Center from Emory College, Georgia, USA (to F.B.M.d.W.) and a grant from Rackham Graduate School at the University of Michigan, USA (to S.D.P.). The authors also thank Y. Lei for assistance with manuscript preparation, and L. Bickel for creating the original brain figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Empathy
-
Any process that emerges from the fact that observers understand others' states by activating personal, neural and mental representations of that state, including the capacity to be affected by and share the emotional state of another; assess the reasons for the other's state; and identify with the other, adopting his or her perspective.
- Theory-of-mind
-
The ability to attribute mental states to others, such as knowledge, intentions and beliefs.
- Affective empathy
-
Also known as emotional empathy. Empathy that is directly affected by the emotional state of another by matching or 'feeling with' it, as a result of perceiving this state.
- Cognitive empathy
-
Empathy derived from a top-down process in which the observer imagines how the target feels, even if the target is not present or their feelings cannot be directly observed.
- Empathic perspective-taking
-
The capacity to take another's affective perspective: for example, understanding their specific situation and needs, separate from one's own, which still requires access to personal representations of the other's state.
- False-belief task
-
A crucial theory-of-mind task that determines whether an observer knows what another knows, even if this knowledge is incongruent with the observer's own.
- Targeted helping
-
Assistance and care based on a cognitive appreciation of the other's specific need or circumstances.
- Tinbergian framework
-
A series of 'why' questions that we may ask about any observed behaviour, as proposed by Niko Tinbergen. The questions concern different levels of causation: for example, why did a behaviour evolve (what are its benefits), why does the behaviour occur (what caused it) and what is the behaviour's phylogenetic origin?
- Emotional contagion
-
Emotional state matching between a target and an observer.
- Consolation behaviour
-
Reassurance behaviour directed at a distressed party, such as a victim of aggression (also see the definition of 'empathic concern').
- Empathic concern
-
Also known as sympathetic concern. Concern about another's state, and attempts to ameliorate this state (also see the definition of 'consolation behaviour').
- Perception–action mechanism
-
Spontaneous activation of an individual's own personal representations for a target, their state and their situation when perceiving the target's state.
- Emotion self-regulation
-
Control over one's own emotions to promote adaptive responding, including response delay, recovery from upsets and selective attention.
- Directed altruism
-
Helping or comforting behaviour directed at an individual in need, pain or distress.
- Convergent evolution
-
A process in which unrelated species independently evolve similar traits or capacities in response to similar environmental pressures.
- Ideomotor action
-
An action that is covertly mimicked when another's action or movement of an object is being observed, such as moving your arm when you watch someone bowl or tilting your head in synchrony with a pendulum swing.
- Motor imitation
-
Re-enactment by the observer of a target's motor movements or facial expressions.
- Affordances
-
Motor or action properties of objects that are activated by their percepts, which are intrinsic to the mental representation of the object.
- Simon effect
-
A behavioural effect in which motor actions are facilitated when they are consistent with the spatial location of the stimulus, and slowed when they are inconsistent or opposing the location.
- Bottom-up
-
Describes a neural and mental process that is stimulus-driven on the basis of directly observed information without requiring explicit cognitive processes or capacities. Empathy arises from bottom-up processes that are shared across species such as motor mimicry, emotional contagion and state matching.
- Top-down
-
Describes a neural and mental process that requires a conscious, cognitive evaluation to take into account information that is not directly observable, such as taking another's perspective or reasoning about their state on the basis of conceptual knowledge. These processes participate in more advanced forms of empathy that are more sophisticated in humans and with age, but they are not required to simply understand how someone feels.
Rights and permissions
About this article
Cite this article
de Waal, F., Preston, S. Mammalian empathy: behavioural manifestations and neural basis. Nat Rev Neurosci 18, 498–509 (2017). https://doi.org/10.1038/nrn.2017.72
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn.2017.72
This article is cited by
-
Social odor choice buffers drug craving
Neuropsychopharmacology (2024)
-
Cortical regulation of helping behaviour towards others in pain
Nature (2024)
-
Does empathy lead to depression? A three-wave random intercept cross-lagged panel analysis in emerging adults
Current Psychology (2024)
-
No browsing, no donating: the impact of title and forwarder on browsing intention of online charity fundraising
International Review on Public and Nonprofit Marketing (2024)
-
Cognitive and affective perspective taking amongst adolescent offenders with variants of callous–unemotional traits
European Child & Adolescent Psychiatry (2024)