Key Points
-
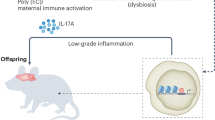
Genetic and environmental risk factors for autism spectrum disorder (ASD) suggest that dysfunction of the immune system may contribute to the development of this disorder.
-
Maternal immune dysfunction due to autoimmune disease, infection or immunogenetics may alter common molecular signalling pathways in the developing brain, increasing the likelihood of ASD.
-
Individuals with ASD exhibit chronic changes in immune system function that may represent disease-related pathophysiology, beneficial compensation or a combination of both.
-
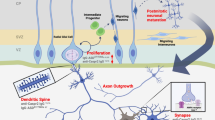
ASD-related changes in the expression of immune molecules in the brain are not always indicative of neuroinflammation, even though these changes may be detrimental to brain development and function.
-
Many immune molecules are expressed in the brain at synapses, and their signalling may converge on several intracellular signalling hubs, such as myocyte-specific enhancer factor 2 (MEF2) and mammalian target of rapamycin (mTOR), that also mediate idiopathic and syndromic forms of ASD.
-
Immune molecules provide a new and important set of targets for the development of new therapeutics for ASD.
Abstract
Increasing evidence points to a central role for immune dysregulation in autism spectrum disorder (ASD). Several ASD risk genes encode components of the immune system and many maternal immune system-related risk factors — including autoimmunity, infection and fetal reactive antibodies — are associated with ASD. In addition, there is evidence of ongoing immune dysregulation in individuals with ASD and in animal models of this disorder. Recently, several molecular signalling pathways — including pathways downstream of cytokines, the receptor MET, major histocompatibility complex class I molecules, microglia and complement factors — have been identified that link immune activation to ASD phenotypes. Together, these findings indicate that the immune system is a point of convergence for multiple ASD-related genetic and environmental risk factors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
King, B. H., Navot, N., Bernier, R. & Webb, S. J. Update on diagnostic classification in autism. Curr. Opin. Psychiatry 27, 105–109 (2014).
Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators & Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years — autism and developmental disabilities monitoring network, 11 Sites, United States, 2010. MMWR Surveill. Summ. 63, 1–21 (2014).
Hertz-Picciotto, I. & Delwiche, L. The rise in autism and the role of age at diagnosis. Epidemiology 20, 84–90 (2009).
Hallmayer, J. et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch. Gen. Psychiatry 68, 1095–1102 (2011). A critical reappraisal of heritability in ASD showing a large role for non-genetic factors.
Rosenberg, R. E. et al. Characteristics and concordance of autism spectrum disorders among 277 twin pairs. Arch. Pediatr. Adolesc. Med. 163, 907–914 (2009).
Pessah, I. N. et al. Immunologic and neurodevelopmental susceptibilities of autism. Neurotoxicology 29, 532–545 (2008).
Bilbo, S. D. & Schwarz, J. M. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front. Behav. Neurosci. 3, 14 (2009). An extensive review of the lifelong consequences of in utero and early postnatal environmental insults on brain and immune function.
Silverstein, A. M. The most elegant immunological experiment of the XIX century. Nat. Immunol. 1, 93–94 (2000).
Jackson, K. D., Howie, L. D. & Akinbami, L. J. Trends in allergic conditions among children: United States, 1997–2011. NCHS Data Brief 121, 1–8 (2013).
Akinbami, L. J. et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief 94, 1–8 (2012).
Thorburn, A. N., Macia, L. & Mackay, C. R. Diet, metabolites, and “Western-lifestyle” inflammatory diseases. Immunity 40, 833–842 (2014).
Stamou, M., Streifel, K. M., Goines, P. E. & Lein, P. J. Neuronal connectivity as a convergent target of gene x environment interactions that confer risk for autism spectrum disorders. Neurotoxicol. Teratol. 36, 3–16 (2013).
Taylor, L. E., Swerdfeger, A. L. & Eslick, G. D. Vaccines are not associated with autism: an evidence-based meta-analysis of case-control and cohort studies. Vaccine 32, 3623–3629 (2014).
Bailey, A. et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol. Med. 25, 63–77 (1995).
Steffenburg, S. et al. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J. Child Psychol. Psychiatry 30, 405–416 (1989).
Sandin, S. et al. The familial risk of autism. JAMA 311, 1770–1777 (2014).
Devlin, B. & Scherer, S. W. Genetic architecture in autism spectrum disorder. Curr. Opin. Genet. Dev. 22, 229–237 (2012).
Gaugler, T. et al. Most genetic risk for autism resides with common variation. Nat. Genet. 46, 881–885 (2014).
Berg, J. M. & Geschwind, D. H. Autism genetics: searching for specificity and convergence. Genome Biol. 13, 247 (2012).
Santini, E. & Klann, E. Reciprocal signaling between translational control pathways and synaptic proteins in autism spectrum disorders. Sci. Signal. 7, re10 (2014).
Franz, D. N. & Weiss, B. D. Molecular therapies for tuberous sclerosis and neurofibromatosis. Curr. Neurol. Neurosci. Rep. 12, 294–301 (2012).
Schaaf, C. P. & Zoghbi, H. Y. Solving the autism puzzle a few pieces at a time. Neuron 70, 806–808 (2011).
Marshall, C. R. et al. Structural variation of chromosomes in autism spectrum disorder. Am. J. Hum. Genet. 82, 477–488 (2008).
O'Roak, B. J. & State, M. W. Autism genetics: strategies, challenges, and opportunities. Autism Res. 1, 4–17 (2008).
Luo, R. et al. Genome-wide transcriptome profiling reveals the functional impact of rare de novo and recurrent CNVs in autism spectrum disorders. Am. J. Hum. Genet. 91, 38–55 (2012).
Peng, Y., Huentelman, M., Smith, C. & Qiu, S. MET receptor tyrosine kinase as an autism genetic risk factor. Int. Rev. Neurobiol. 113, 135–165 (2013).
Campbell, D. B. et al. Disruption of cerebral cortex MET signaling in autism spectrum disorder. Ann. Neurol. 62, 243–250 (2007).
Campbell, D. B., Li, C., Sutcliffe, J. S., Persico, A. M. & Levitt, P. Genetic evidence implicating multiple genes in the MET receptor tyrosine kinase pathway in autism spectrum disorder. Autism Res. 1, 159–168 (2008).
Jackson, P. B. et al. Further evidence that the rs1858830 C variant in the promoter region of the MET gene is associated with autistic disorder. Autism Res. 2, 232–236 (2009).
Voineagu, I. et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 474, 380–384 (2011). A landmark study identifying the enrichment of synaptic and immune gene modules in the transcriptomes of individuals with ASD.
Rudie, J. D. et al. Autism-associated promoter variant in MET impacts functional and structural brain networks. Neuron 75, 904–915 (2012).
Hedrick, A. et al. Autism risk gene MET variation and cortical thickness in typically developing children and adolescents. Autism Res. 5, 434–439 (2012).
Plummer, J. T. et al. Transcriptional regulation of the MET receptor tyrosine kinase gene by MeCP2 and sex-specific expression in autism and Rett syndrome. Transl Psychiatry 3, e316 (2013).
Rutella, S. et al. Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (IL)-10++IL-12low/neg accessory cells with dendritic-cell features. Blood 108, 218–227 (2006).
Okunishi, K. et al. A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function. J. Immunol. 175, 4745–4753 (2005).
Tahara, Y. et al. Hepatocyte growth factor facilitates colonic mucosal repair in experimental ulcerative colitis in rats. J. Pharmacol. Exp. Ther. 307, 146–151 (2003).
Ido, A., Numata, M., Kodama, M. & Tsubouchi, H. Mucosal repair and growth factors: recombinant human hepatocyte growth factor as an innovative therapy for inflammatory bowel disease. J. Gastroenterol. 40, 925–931 (2005).
Okunishi, K. et al. Hepatocyte growth factor significantly suppresses collagen-induced arthritis in mice. J. Immunol. 179, 5504–5513 (2007).
Futamatsu, H. et al. Hepatocyte growth factor ameliorates the progression of experimental autoimmune myocarditis: a potential role for induction of T helper 2 cytokines. Circ. Res. 96, 823–830 (2005).
Kuroiwa, T. et al. Hepatocyte growth factor prevents lupus nephritis in a murine lupus model of chronic graft-versus-host disease. Arthritis Res. Ther. 8, R123 (2006).
Oh, K. et al. Ameliorating effect of hepatocyte growth factor on inflammatory bowel disease in a murine model. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G729–G735 (2005).
Mizuno, S., Matsumoto, K., Li, M. Y. & Nakamura, T. HGF reduces advancing lung fibrosis in mice: a potential role for MMP-dependent myofibroblast apoptosis. FASEB J. 19, 580–582 (2005).
Heuer, L., Braunschweig, D., Ashwood, P., Van de Water, J. & Campbell, D. B. Association of a MET genetic variant with autism-associated maternal autoantibodies to fetal brain proteins and cytokine expression. Translat. Psychiatry 1, e48 (2011).
Thaxton, J. E. & Sharma, S. Interleukin-10: a multi-faceted agent of pregnancy. Am. J. Reprod. Immunol. 63, 482–491 (2010).
Volk, H. E. et al. Autism spectrum disorder: interaction of air pollution with the MET receptor tyrosine kinase gene. Epidemiology 25, 44–47 (2014). A proof-of-concept study showing how gene–environment synergies may contribute to an increase in the incidence of ASD.
Hsiao, E. Y. Immune dysregulation in autism spectrum disorder. Int. Rev. Neurobiol. 113, 269–302 (2013).
Needleman, L. A. & McAllister, A. K. The major histocompatibility complex and autism spectrum disorder. Dev. Neurobiol. 72, 1288–1301 (2012).
Gough, S. C. & Simmonds, M. J. The HLA region and autoimmune disease: associations and mechanisms of action. Curr. Genom. 8, 453–465 (2007).
Keil, A. et al. Parental autoimmune diseases associated with autism spectrum disorders in offspring. Epidemiology 21, 805–808 (2010).
Mostafa, G. A., Shehab, A. A. & Al-Ayadhi, L. Y. The link between some alleles on human leukocyte antigen system and autism in children. J. Neuroimmunol. 255, 70–74 (2013).
Warren, R. P., Burger, R. A., Odell, D., Torres, A. R. & Warren, W. L. Decreased plasma concentrations of the C4B complement protein in autism. Arch. Pediatr. Adolesc. Med. 148, 180–183 (1994).
Warren, R. P. et al. Increased frequency of the null allele at the complement C4b locus in autism. Clin. Exp. Immunol. 83, 438–440 (1991).
Deng, Y. & Tsao, B. P. Genetic susceptibility to systemic lupus erythematosus in the genomic era. Nat. Rev. Rheumatol. 6, 683–692 (2010).
Mostafa, G. A. & Shehab, A. A. The link of C4B null allele to autism and to a family history of autoimmunity in Egyptian autistic children. J. Neuroimmunol. 223, 115–119 (2010).
Careaga, M. & Ashwood, P. Autism spectrum disorders: from immunity to behavior. Methods Mol. Biol. 934, 219–240 (2012).
O'Roak, B. J. et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat. Genet. 43, 585–589 (2011).
Sanders, S. J. et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485, 237–241 (2012).
Sauna, Z. E. & Kimchi-Sarfaty, C. Understanding the contribution of synonymous mutations to human disease. Nat. Rev. Genet. 12, 683–691 (2011).
Parmley, J. L. & Hurst, L. D. How do synonymous mutations affect fitness? Bioessays 29, 515–519 (2007).
Takahashi, H. & Craig, A. M. Protein tyrosine phosphatases PTPδ, PTPσ, and LAR: presynaptic hubs for synapse organization. Trends Neurosci. 36, 522–534 (2013).
Bhat, S. S. et al. Disruption of the IL1RAPL1 gene associated with a pericentromeric inversion of the X chromosome in a patient with mental retardation and autism. Clin. Genet. 73, 94–96 (2008).
Bahi, N. et al. IL1 receptor accessory protein like, a protein involved in X-linked mental retardation, interacts with neuronal calcium sensor-1 and regulates exocytosis. Hum. Mol. Genet. 12, 1415–1425 (2003).
Piton, A. et al. Mutations in the calcium-related gene IL1RAPL1 are associated with autism. Hum. Mol. Genet. 17, 3965–3974 (2008).
McDougle, C. J. et al. Toward an immune-mediated subtype of autism spectrum disorder. Brain Res. 1617, 72–92 (2014).
Atladottir, H. O. et al. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics 124, 687–694 (2009).
Kohane, I. S. et al. The co-morbidity burden of children and young adults with autism spectrum disorders. PLoS ONE 7, e33224 (2012).
Patterson, P. H. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav. Brain Res. 204, 313–321 (2009). An authoritative review of immune contributions to neurodevelopmental disorders.
Iaccarino, L. et al. Polarization of TH2 response is decreased during pregnancy in systemic lupus erythematosus. Reumatismo 64, 314–320 (2012).
Diamond, B., Huerta, P. T., Mina-Osorio, P., Kowal, C. & Volpe, B. T. Losing your nerves? Maybe it's the antibodies. Nat. Rev. Immunol. 9, 449–456 (2009). An excellent review of the mechanisms by which autoantibodies modulate brain function and behaviour.
Brimberg, L., Sadiq, A., Gregersen, P. K. & Diamond, B. Brain-reactive IgG correlates with autoimmunity in mothers of a child with an autism spectrum disorder. Mol. Psychiatry 18, 1171–1177 (2013).
Lee, J. Y. et al. Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus. Nat. Med. 15, 91–96 (2009).
Zhang, J., Jacobi, A. M., Wang, T. & Diamond, B. Pathogenic autoantibodies in systemic lupus erythematosus are derived from both self-reactive and non-self-reactive B cells. Mol. Med. 14, 675–681 (2008).
Franchin, G. et al. Anti-DNA antibodies cross-react with C1q. J. Autoimmun. 44, 34–39 (2013).
Wang, L. et al. Female mouse fetal loss mediated by maternal autoantibody. J. Exp. Med. 209, 1083–1089 (2012). The first study to demonstrate a molecular mechanism whereby maternally derived autoantibodies exert sex-specific effects.
Frazier, T. W., Georgiades, S., Bishop, S. L. & Hardan, A. Y. Behavioral and cognitive characteristics of females and males with autism in the Simons Simplex Collection. J. Am. Acad. Child Adolesc. Psychiatry 53, 329–340 (2014).
Braunschweig, D. & Van de Water, J. Maternal autoantibodies in autism. Arch. Neurol. 69, 693–699 (2012).
Braunschweig, D. et al. Autism-specific maternal autoantibodies recognize critical proteins in developing brain. Transl Psychiatry 3, e277 (2013). A seminal study identifying fetal protein targets of ASD-specific maternal antibodies.
Martin, L. A. et al. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav. Immun. 22, 806–816 (2008). The first study to show a causal relationship between antibodies from mothers of ASD children and ASD-like behaviours in a non-human primate model.
Bauman, M. D. et al. Maternal antibodies from mothers of children with autism alter brain growth and social behavior development in the rhesus monkey. Transl Psychiatry 3, e278 (2013).
Ben Bashat, D. et al. Accelerated maturation of white matter in young children with autism: a high b value DWI study. Neuroimage 37, 40–47 (2007).
Billeci, L., Calderoni, S., Tosetti, M., Catani, M. & Muratori, F. White matter connectivity in children with autism spectrum disorders: a tract-based spatial statistics study. BMC Neurol. 12, 148 (2012).
Wolff, J. J. et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am. J. Psychiatry 169, 589–600 (2012).
Singer, H. S. et al. Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: a pregnant dam mouse model. J. Neuroimmunol. 211, 39–48 (2009).
Camacho, J. et al. Embryonic intraventricular exposure to autism-specific maternal autoantibodies produces alterations in autistic-like stereotypical behaviors in offspring mice. Behav. Brain Res. 266, 46–51 (2014).
Patterson, P. H. Maternal infection and immune involvement in autism. Trends Mol. Med. 17, 389–394 (2011).
Chess, S. Autism in children with congenital rubella. J. Autism Child. Schizophr. 1, 33–47 (1971). The first report of increased incidence of ASD using specific behavioural criteria in a large cohort of children with congenital rubella.
Chess, S. Follow-up report on autism in congenital rubella. J. Autism Child. Schizophr. 7, 69–81 (1977).
Swisher, C. N. & Swisher, L. Letter: congenital rubella and autistic behavior. N. Engl. J. Med. 293, 198 (1975).
Abdallah, M. W. et al. Amniotic fluid inflammatory cytokines: potential markers of immunologic dysfunction in autism spectrum disorders. World J. Biol. Psychiatry 14, 528–538 (2013).
Goines, P. E. et al. Increased midgestational IFN-γ, IL-4 and IL-5 in women bearing a child with autism: a case-control study. Mol. Autism 2, 13 (2011).
Atladottir, H. O. et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J. Autism Dev. Disord. 40, 1423–1430 (2010).
Atladottir, H. O., Henriksen, T. B., Schendel, D. E. & Parner, E. T. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics 130, e1447–e1454 (2012). The largest epidemiological study so far on ASD incidence following maternal infection.
Missault, S. et al. The risk for behavioural deficits is determined by the maternal immune response to prenatal immune challenge in a neurodevelopmental model. Brain Behav. Immun. 42, 138–46 (2014).
Patterson, P. H. Maternal infection: window on neuroimmune interactions in fetal brain development and mental illness. Curr. Opin. Neurobiol. 12, 115–118 (2002).
Knuesel, I. et al. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 10, 643–660 (2014). A comprehensive review of empirical evidence linking MIA to numerous CNS disorders.
Smith, S. E., Li, J., Garbett, K., Mirnics, K. & Patterson, P. H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 27, 10695–10702 (2007).
Malkova, N. V., Yu, C. Z., Hsiao, E. Y., Moore, M. J. & Patterson, P. H. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav. Immun. 26, 607–616 (2012). The first report to demonstrate face and construct validity of the poly(I:C) rodent model of MIA for the three core symptoms of ASD.
Bauman, M. D. et al. Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biol. Psychiatry 75, 332–341 (2014).
Shi, L. et al. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav. Immun. 23, 116–123 (2009).
Amaral, D. G., Schumann, C. M. & Nordahl, C. W. Neuroanatomy of autism. Trends Neurosci. 31, 137–145 (2008).
Ponzio, N. M., Servatius, R., Beck, K., Marzouk, A. & Kreider, T. Cytokine levels during pregnancy influence immunological profiles and neurobehavioral patterns of the offspring. Ann. NY Acad. Sci. 1107, 118–128 (2007).
Meyer, U. et al. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol. Psychiatry 13, 208–221 (2008).
Onore, C., Careaga, M. & Ashwood, P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav. Immun. 26, 383–392 (2012).
Mostafa, G. A. & Al-Ayadhi, L. Y. The possible relationship between allergic manifestations and elevated serum levels of brain specific auto-antibodies in autistic children. J. Neuroimmunol. 261, 77–81 (2013).
Singh, V. K., Warren, R., Averett, R. & Ghaziuddin, M. Circulating autoantibodies to neuronal and glial filament proteins in autism. Pediatr. Neurol. 17, 88–90 (1997).
Singh, V. K., Warren, R. P., Odell, J. D., Warren, W. L. & Cole, P. Antibodies to myelin basic protein in children with autistic behavior. Brain Behav. Immun. 7, 97–103 (1993).
Vojdani, A. et al. Antibodies to neuron-specific antigens in children with autism: possible cross-reaction with encephalitogenic proteins from milk, Chlamydia pneumoniae and Streptococcus group A. J. Neuroimmunol. 129, 168–177 (2002).
Singer, H. S. et al. Antibrain antibodies in children with autism and their unaffected siblings. J. Neuroimmunol. 178, 149–155 (2006).
Singh, V. K., Singh, E. A. & Warren, R. P. Hyperserotoninemia and serotonin receptor antibodies in children with autism but not mental retardation. Biol. Psychiatry 41, 753–755 (1997).
Singh, V. K. & Rivas, W. H. Prevalence of serum antibodies to caudate nucleus in autistic children. Neurosci. Lett. 355, 53–56 (2004).
Silva, S. C. et al. Autoantibody repertoires to brain tissue in autism nuclear families. J. Neuroimmunol. 152, 176–182 (2004).
Cabanlit, M., Wills, S., Goines, P., Ashwood, P. & Van de Water, J. Brain-specific autoantibodies in the plasma of subjects with autistic spectrum disorder. Ann. NY Acad. Sci. 1107, 92–103 (2007).
Wills, S. et al. Detection of autoantibodies to neural cells of the cerebellum in the plasma of subjects with autism spectrum disorders. Brain Behav. Immun. 23, 64–74 (2009).
Morris, C. M., Zimmerman, A. W. & Singer, H. S. Childhood serum anti-fetal brain antibodies do not predict autism. Pediatr. Neurol. 41, 288–290 (2009).
Goines, P. et al. Autoantibodies to cerebellum in children with autism associate with behavior. Brain Behav. Immun. 25, 514–523 (2011).
Vargas, D. L., Nascimbene, C., Krishnan, C., Zimmerman, A. W. & Pardo, C. A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 57, 67–81 (2005).
Molloy, C. A. et al. Elevated cytokine levels in children with autism spectrum disorder. J. Neuroimmunol. 172, 198–205 (2006).
Okada, K. et al. Decreased serum levels of transforming growth factor-β1 in patients with autism. Prog. Neuropsychopharmacol. Biol. Psychiatry 31, 187–190 (2007).
Ashwood, P. et al. Decreased transforming growth factor β1 in autism: a potential link between immune dysregulation and impairment in clinical behavioral outcomes. J. Neuroimmunol. 204, 149–153 (2008).
Ashwood, P. et al. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 25, 40–45 (2011).
Ashwood, P. et al. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J. Neuroimmunol. 232, 196–199 (2011).
Abdallah, M. W. et al. Neonatal levels of cytokines and risk of autism spectrum disorders: an exploratory register-based historic birth cohort study utilizing the Danish Newborn Screening Biobank. J. Neuroimmunol. 252, 75–82 (2012).
Napolioni, V. et al. Plasma cytokine profiling in sibling pairs discordant for autism spectrum disorder. J. Neuroinflammation 10, 38 (2013).
Warren, R. P., Foster, A. & Margaretten, N. C. Reduced natural killer cell activity in autism. J. Am. Acad. Child Adolesc. Psychiatry 26, 333–335 (1987).
Enstrom, A. M., Onore, C. E., Van de Water, J. A. & Ashwood, P. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav. Immun. 24, 64–71 (2010).
Warren, R. P., Margaretten, N. C., Pace, N. C. & Foster, A. Immune abnormalities in patients with autism. J. Autism Dev. Disord. 16, 189–197 (1986).
Ashwood, P. et al. Altered T cell responses in children with autism. Brain Behav. Immun. 25, 840–849 (2011).
Gupta, S., Aggarwal, S., Rashanravan, B. & Lee, T. Th1- and Th2-like cytokines in CD4+ and CD8+ T cells in autism. J. Neuroimmunol. 85, 106–109 (1998).
Warren, R. P., Yonk, J., Burger, R. W., Odell, D. & Warren, W. L. DR-positive T cells in autism: association with decreased plasma levels of the complement C4B protein. Neuropsychobiology 31, 53–57 (1995).
Stubbs, E. G. & Crawford, M. L. Depressed lymphocyte responsiveness in autistic children. J. Autism Child. Schizophr. 7, 49–55 (1977). The first report to identify immune cell abnormalities in individuals with ASD.
Plioplys, A. V., Greaves, A., Kazemi, K. & Silverman, E. Lymphocyte function in autism and Rett syndrome. Neuropsychobiology 29, 12–16 (1994).
Derecki, N. C. et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J. Exp. Med. 207, 1067–1080 (2010).
Hsiao, E. Y., McBride, S. W., Chow, J., Mazmanian, S. K. & Patterson, P. H. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc. Natl Acad. Sci. USA 109, 12776–12781 (2012).
Mandal, M. et al. Maternal immune stimulation during pregnancy shapes the immunological phenotype of offspring. Brain Behav. Immun. 33, 33–45 (2013).
Onore, C. E., Schwartzer, J. J., Careaga, M., Berman, R. F. & Ashwood, P. Maternal immune activation leads to activated inflammatory macrophages in offspring. Brain Behav. Immun. 38, 220–226 (2014).
Li, X. et al. Elevated immune response in the brain of autistic patients. J. Neuroimmunol. 207, 111–116 (2009).
Wei, H. et al. IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. J. Neuroinflammation 8, 52 (2011).
Morgan, J. T. et al. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol. Psychiatry 68, 368–376 (2010).
Tetreault, N. A. et al. Microglia in the cerebral cortex in autism. J. Autism Dev. Disord. 42, 2569–2584 (2012).
Tani, Y., Fernell, E., Watanabe, Y., Kanai, T. & Langstrom, B. Decrease in 6R-5,6,7,8-tetrahydrobiopterin content in cerebrospinal fluid of autistic patients. Neurosci. Lett. 181, 169–172 (1994).
Komori, H. et al. Cerebrospinal fluid biopterin and biogenic amine metabolites during oral R-THBP therapy for infantile autism. J. Autism Dev. Disord. 25, 183–193 (1995).
Zimmerman, A. W. et al. Cerebrospinal fluid and serum markers of inflammation in autism. Pediatr. Neurol. 33, 195–201 (2005).
Arrode-Bruses, G. & Bruses, J. L. Maternal immune activation by poly I:C induces expression of cytokines IL-1β and IL-13, chemokine MCP-1 and colony stimulating factor VEGF in fetal mouse brain. J. Neuroinflammation 9, 83 (2012).
Meyer, U. et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J. Neurosci. 26, 4752–4762 (2006).
Garay, P. A., Hsiao, E. Y., Patterson, P. H. & McAllister, A. K. Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav. Immun. 31, 54–68 (2013). The first study to show age- and region-specific immune changes in the brains of offspring from MIA rodents.
Estes, M. L. & McAllister, A. K. Alterations in immune cells and mediators in the brain: it's not always neuroinflammation! Brain Pathol. 24, 623–630 (2014).
Palmer, C., Bik, E. M., DiGiulio, D. B., Relman, D. A. & Brown, P. O. Development of the human infant intestinal microbiota. PLoS Biol. 5, e177 (2007).
Wu, H. J. & Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 3, 4–14 (2012).
Salzman, N. H. Microbiota–immune system interaction: an uneasy alliance. Curr. Opin. Microbiol. 14, 99–105 (2011).
Song, Y., Liu, C. & Finegold, S. M. Real-time PCR quantitation of clostridia in feces of autistic children. Appl. Environ. Microbiol. 70, 6459–6465 (2004).
Parracho, H. M., Bingham, M. O., Gibson, G. R. & McCartney, A. L. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J. Med. Microbiol. 54, 987–991 (2005).
Finegold, S. M. Desulfovibrio species are potentially important in regressive autism. Med. Hypotheses 77, 270–274 (2011).
Gaboriau-Routhiau, V. et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31, 677–689 (2009).
Ishikawa, H. et al. Effect of intestinal microbiota on the induction of regulatory CD25+ CD4+ T cells. Clin. Exp. Immunol. 153, 127–135 (2008).
Mazmanian, S. K., Liu, C. H., Tzianabos, A. O. & Kasper, D. L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122, 107–118 (2005).
de Magistris, L. et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J. Pediatr. Gastroenterol. Nutr. 51, 418–424 (2010).
Coury, D. L. et al. Gastrointestinal conditions in children with autism spectrum disorder: developing a research agenda. Pediatrics 130 (Suppl. 2), S160–S168 (2012).
Hsiao, E. Y. et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463 (2013). A proof-of-concept study showing that ASD-like behaviours can be ameliorated by modulation of the microbiota.
Finegold, S. M., Downes, J. & Summanen, P. H. Microbiology of regressive autism. Anaerobe 18, 260–262 (2012).
Deverman, B. E. & Patterson, P. H. Cytokines and CNS development. Neuron 64, 61–78 (2009). An excellent review of the diverse and non-classical roles of cytokines in the developing brain.
Garay, P. A. & McAllister, A. K. Novel roles for immune molecules in neural development: implications for neurodevelopmental disorders. Front. Synaptic Neurosci. 2, 136 (2010).
Goines, P. E. & Ashwood, P. Cytokine dysregulation in autism spectrum disorders (ASD): possible role of the environment. Neurotoxicol. Teratol. 36, 67–81 (2013).
de la Mano, A. et al. Role of interleukin-1β in the control of neuroepithelial proliferation and differentiation of the spinal cord during development. Cytokine 37, 128–137 (2007).
Gambino, F. et al. IL1RAPL1 controls inhibitory networks during cerebellar development in mice. Eur. J. Neurosci. 30, 1476–1486 (2009).
Valnegri, P. et al. The X-linked intellectual disability protein IL1RAPL1 regulates excitatory synapse formation by binding PTPδ and RhoGAP2. Hum. Mol. Genet. 20, 4797–4809 (2011).
Yoshida, T. & Mishina, M. Zebrafish orthologue of mental retardation protein IL1RAPL1 regulates presynaptic differentiation. Mol. Cell. Neurosci. 39, 218–228 (2008).
Yoshida, T. et al. IL-1 receptor accessory protein-like 1 associated with mental retardation and autism mediates synapse formation by trans-synaptic interaction with protein tyrosine phosphatase δ. J. Neurosci. 31, 13485–13499 (2011).
Yoshida, T. et al. Interleukin-1 receptor accessory protein organizes neuronal synaptogenesis as a cell adhesion molecule. J. Neurosci. 32, 2588–2600 (2012).
Pavlowsky, A. et al. A postsynaptic signaling pathway that may account for the cognitive defect due to IL1RAPL1 mutation. Curr. Biol. 20, 103–115 (2010).
Houbaert, X. et al. Target-specific vulnerability of excitatory synapses leads to deficits in associative memory in a model of intellectual disorder. J. Neurosci. 33, 13805–13819 (2013).
Moretti, P. et al. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J. Neurosci. 26, 319–327 (2006).
Goshen, I. & Yirmiya, R. Interleukin-1 (IL-1): a central regulator of stress responses. Front. Neuroendocrinol. 30, 30–45 (2009).
Bernardino, L. et al. Tumor necrosis factor-α modulates survival, proliferation, and neuronal differentiation in neonatal subventricular zone cell cultures. Stem Cells 26, 2361–2371 (2008).
Pribiag, H. & Stellwagen, D. Neuroimmune regulation of homeostatic synaptic plasticity. Neuropharmacology 78, 13–22 (2014).
Nagakura, I., Van Wart, A., Petravicz, J., Tropea, D. & Sur, M. STAT1 regulates the homeostatic component of visual cortical plasticity via an AMPA receptor-mediated mechanism. J. Neurosci. 34, 10256–10263 (2014).
Murray, P. J. The JAK–STAT signaling pathway: input and output integration. J. Immunol. 178, 2623–2629 (2007).
Tropea, D. et al. Gene expression changes and molecular pathways mediating activity-dependent plasticity in visual cortex. Nat. Neurosci. 9, 660–668 (2006).
Tai, D. J., Hsu, W. L., Liu, Y. C., Ma, Y. L. & Lee, E. H. Novel role and mechanism of protein inhibitor of activated STAT1 in spatial learning. EMBO J. 30, 205–220 (2011).
Feng, J. et al. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat. Neurosci. 13, 423–430 (2010).
Nicolas, C. S. et al. The Jak/STAT pathway is involved in synaptic plasticity. Neuron 73, 374–390 (2012).
Judson, M. C., Bergman, M. Y., Campbell, D. B., Eagleson, K. L. & Levitt, P. Dynamic gene and protein expression patterns of the autism-associated met receptor tyrosine kinase in the developing mouse forebrain. J. Comp. Neurol. 513, 511–531 (2009).
Ieraci, A., Forni, P. E. & Ponzetto, C. Viable hypomorphic signaling mutant of the Met receptor reveals a role for hepatocyte growth factor in postnatal cerebellar development. Proc. Natl Acad. Sci. USA 99, 15200–15205 (2002).
Wu, H. H. & Levitt, P. Prenatal expression of MET receptor tyrosine kinase in the fetal mouse dorsal raphe nuclei and the visceral motor/sensory brainstem. Dev. Neurosci. 35, 1–16 (2013).
Eagleson, K. L., Milner, T. A., Xie, Z. & Levitt, P. Synaptic and extrasynaptic location of the receptor tyrosine kinase Met during postnatal development in the mouse neocortex and hippocampus. J. Comp. Neurol. 521, 3241–3259 (2013).
Tyndall, S. J. & Walikonis, R. S. The receptor tyrosine kinase Met and its ligand hepatocyte growth factor are clustered at excitatory synapses and can enhance clustering of synaptic proteins. Cell Cycle 5, 1560–1568 (2006).
Nakano, M. et al. Hepatocyte growth factor promotes the number of PSD-95 clusters in young hippocampal neurons. Exp. Neurol. 207, 195–202 (2007).
Kawas, L. H., Benoist, C. C., Harding, J. W., Wayman, G. A. & Abu-Lail, N. I. Nanoscale mapping of the Met receptor on hippocampal neurons by AFM and confocal microscopy. Nanomed. Nanotechnol. Biol. Med. 9, 428–438 (2013).
Lim, C. S. & Walikonis, R. S. Hepatocyte growth factor and c-Met promote dendritic maturation during hippocampal neuron differentiation via the Akt pathway. Cell. Signal. 20, 825–835 (2008).
Akimoto, M. et al. Hepatocyte growth factor as an enhancer of NMDA currents and synaptic plasticity in the hippocampus. Neuroscience 128, 155–162 (2004).
Qiu, S., Anderson, C. T., Levitt, P. & Shepherd, G. M. Circuit-specific intracortical hyperconnectivity in mice with deletion of the autism-associated Met receptor tyrosine kinase. J. Neurosci. 31, 5855–5864 (2011).
Elmer, B. M., Estes, M. L., Barrow, S. L. & McAllister, A. K. MHCI requires MEF2 transcription factors to negatively regulate synapse density during development and in disease. J. Neurosci. 33, 13791–13804 (2013).
Corriveau, R. A., Huh, G. S. & Shatz, C. J. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron 21, 505–520 (1998). The first report of the activity-dependent expression of MHCI molecules in the developing brain.
Elmer, B. M. & McAllister, A. K. Major histocompatibility complex class I proteins in brain development and plasticity. Trends Neurosci. 35, 660–670 (2012).
Lee, H. et al. Synapse elimination and learning rules co-regulated by MHC class I H2-Db. Nature 509, 195–200 (2014).
Goddard, C. A., Butts, D. A. & Shatz, C. J. Regulation of CNS synapses by neuronal MHC class I. Proc. Natl Acad. Sci. USA 104, 6828–6833 (2007).
Needleman, L. A., Liu, X. B., El-Sabeawy, F., Jones, E. G. & McAllister, A. K. MHC class I molecules are present both pre- and postsynaptically in the visual cortex during postnatal development and in adulthood. Proc. Natl Acad. Sci. USA 107, 16999–17004 (2010).
Glynn, M. W. et al. MHCI negatively regulates synapse density during the establishment of cortical connections. Nat. Neurosci. 14, 442–451 (2011).
Fourgeaud, L. et al. MHC class I modulates NMDA receptor function and AMPA receptor trafficking. Proc. Natl Acad. Sci. USA 107, 22278–22283 (2010).
Huh, G. S. et al. Functional requirement for class I MHC in CNS development and plasticity. Science 290, 2155–2159 (2000).
Nelson, P. A. et al. MHC class I immune proteins are critical for hippocampus-dependent memory and gate NMDAR-dependent hippocampal long-term depression. Learn. Mem. 20, 505–517 (2013).
Flavell, S. W. et al. Genome-wide analysis of MEF2 transcriptional program reveals synaptic target genes and neuronal activity-dependent polyadenylation site selection. Neuron 60, 1022–1038 (2008).
Flavell, S. W. et al. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science 311, 1008–1012 (2006).
Paciorkowski, A. R. et al. MEF2C haploinsufficiency features consistent hyperkinesis, variable epilepsy, and has a role in dorsal and ventral neuronal developmental pathways. Neurogenetics 14, 99–111 (2013).
Martin, C. L. et al. Cytogenetic and molecular characterization of A2BP1/FOX1 as a candidate gene for autism. Am. J.Med. Genet. B Neuropsychiatr. Genet. 144B, 869–876 (2007).
Babatz, T. D., Kumar, R. A., Sudi, J., Dobyns, W. B. & Christian, S. L. Copy number and sequence variants implicate APBA2 as an autism candidate gene. Autism Res. 2, 359–364 (2009).
Parikshak, N. N. et al. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell 155, 1008–1021 (2013).
Zang, T. et al. Postsynaptic FMRP bidirectionally regulates excitatory synapses as a function of developmental age and MEF2 activity. Mol. Cell. Neurosci. 56, 39–49 (2013).
Tsai, N. P. et al. Multiple autism-linked genes mediate synapse elimination via proteasomal degradation of a synaptic scaffold PSD-95. Cell 151, 1581–1594 (2012).
Ebert, D. H. & Greenberg, M. E. Activity-dependent neuronal signalling and autism spectrum disorder. Nature 493, 327–337 (2013).
Morgan, J. T. et al. Abnormal microglial-neuronal spatial organization in the dorsolateral prefrontal cortex in autism. Brain Res. 1456, 72–81 (2012).
Suzuki, K. et al. Microglial activation in young adults with autism spectrum disorder. JAMA Psychiatry 70, 49–58 (2013).
Prinz, M. & Priller, J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat. Rev. Neurosci. 15, 300–312 (2014). An authoritative review of microglial origins and contributions to normal brain function and disease.
Butovsky, O. et al. Microglia activated by IL-4 or IFN-γ differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol. Cell. Neurosci. 31, 149–160 (2006).
Cunningham, C. L., Martinez-Cerdeno, V. & Noctor, S. C. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. 33, 4216–4233 (2013).
Schafer, D. P., Lehrman, E. K. & Stevens, B. The “quad-partite” synapse: microglia-synapse interactions in the developing and mature CNS. Glia 61, 24–36 (2013).
Paolicelli, R. C. et al. Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458 (2011).
Zhan, Y. et al. Deficient neuron–microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 17, 400–406 (2014).
Odell, D. et al. Confirmation of the association of the C4B null allelle in autism. Hum. Immunol. 66, 140–145 (2005).
Truedsson, L., Bengtsson, A. A. & Sturfelt, G. Complement deficiencies and systemic lupus erythematosus. Autoimmunity 40, 560–566 (2007).
Stevens, B. et al. The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178 (2007). A pioneering report of the complement cascade playing an unexpected part in synaptic pruning.
Gasque, P. Complement: a unique innate immune sensor for danger signals. Mol. Immunol. 41, 1089–1098 (2004).
Schafer, D. P. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012).
Bialas, A. R. & Stevens, B. TGF-β signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat. Neurosci. 16, 1773–1782 (2013).
Bilbo, S. D. & Frank, A. Beach award: programming of neuroendocrine function by early-life experience: a critical role for the immune system. Horm. Behav. 63, 684–691 (2013).
Perry, V. H., Newman, T. A. & Cunningham, C. The impact of systemic infection on the progression of neurodegenerative disease. Nat. Rev. Neurosci. 4, 103–112 (2003).
Streit, W. J. & Xue, Q. S. Life and death of microglia. J. Neuroimmune Pharmacol. 4, 371–379 (2009).
Costa-Mattioli, M. & Monteggia, L. M. mTOR complexes in neurodevelopmental and neuropsychiatric disorders. Nat. Neurosci. 16, 1537–1543 (2013).
Takei, N. & Nawa, H. mTOR signaling and its roles in normal and abnormal brain development. Front. Mol. Neurosci. 7, 28 (2014).
Kassai, H. et al. Selective activation of mTORC1 signaling recapitulates microcephaly, tuberous sclerosis, and neurodegenerative diseases. Cell Rep. 7, 1626–1639 (2014).
Powell, J. D., Pollizzi, K. N., Heikamp, E. B. & Horton, M. R. Regulation of immune responses by mTOR. Annu. Rev. Immunol. 30, 39–68 (2012).
Ricciardi, S. et al. Reduced AKT/mTOR signaling and protein synthesis dysregulation in a Rett syndrome animal model. Hum. Mol. Genet. 20, 1182–1196 (2011).
Qin, S. et al. Failure to ubiquitinate c-Met leads to hyperactivation of mTOR signaling in a mouse model of autosomal dominant polycystic kidney disease. J. Clin. Invest. 120, 3617–3628 (2010).
Derecki, N. C. et al. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature 484, 105–109 (2012). A seminal study showing immune cell-specific rescue of Rett syndrome-like behaviours and pathophysiology postnatally in a mouse model.
Sharma, A. et al. Autologous bone marrow mononuclear cell therapy for autism: an open label proof of concept study. Stem Cells Int. 2013, 623875 (2013).
Lv, Y. T. et al. Transplantation of human cord blood mononuclear cells and umbilical cord-derived mesenchymal stem cells in autism. J. Translat. Med. 11, 196 (2013).
Bilousova, T. V. et al. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J. Med. Genet. 46, 94–102 (2009).
Rotschafer, S. E., Trujillo, M. S., Dansie, L. E., Ethell, I. M. & Razak, K. A. Minocycline treatment reverses ultrasonic vocalization production deficit in a mouse model of fragile X syndrome. Brain Res. 1439, 7–14 (2012).
Leigh, M. J. et al. A randomized double-blind, placebo-controlled trial of minocycline in children and adolescents with fragile X syndrome. J. Dev. Behav. Pediatr. 34, 147–155 (2013).
Posey, D. J. et al. A pilot study of D-cycloserine in subjects with autistic disorder. Am. J. Psychiatry 161, 2115–2117 (2004).
Sandler, R. H. et al. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J. Child Neurol. 15, 429–435 (2000).
Ramirez, P. L., Barnhill, K., Gutierrez, A., Schutte, C. & Hewitson, L. Improvements in behavioral symptoms following antibiotic therapy in a 14-year-old male with autism. Case Rep. Psychiatry 2013, 239034 (2013).
Pardo, C. A. et al. A pilot open-label trial of minocycline in patients with autism and regressive features. J. Neurodev. Disord. 5, 9 (2013).
Jung, H. J. et al. Minocycline inhibits angiogenesis in vitro through the translational suppression of HIF-1α. Arch. Biochem. Biophys. 545, 74–82 (2014).
Sullivan, R. C. Why do autistic children...? J. Autism Dev. Disord. 10, 231–241 (1980).
Cotterill, R. M. Fever in autistics. Nature 313, 426 (1985).
Curran, L. K. et al. Behaviors associated with fever in children with autism spectrum disorders. Pediatrics 120, e1386–e1392 (2007).
Naviaux, J. C. et al. Reversal of autism-like behaviors and metabolism in adult mice with single-dose antipurinergic therapy. Transl Psychiatry 4, e400 (2014).
Naviaux, J. C. et al. Antipurinergic therapy corrects the autism-like features in the fragile X (Fmr1 knockout) mouse model. Mol. Autism 6, 1 (2015).
Kipnis, J., Gadani, S. & Derecki, N. C. Pro-cognitive properties of T cells. Nat. Rev. Immunol. 12, 663–669 (2012). An excellent review examining the unexpected physiological role of adaptive immunity in learning and memory.
Walsh, J. T., Watson, N. & Kipnis, J. T cells in the central nervous system: messengers of destruction or purveyors of protection? Immunology 141, 340–344 (2014).
Kipnis, J., Cohen, H., Cardon, M., Ziv, Y. & Schwartz, M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc. Natl Acad. Sci. USA 101, 8180–8185 (2004).
Rattazzi, L. et al. CD4+ but not CD8+ T cells revert the impaired emotional behavior of immunocompromised RAG-1-deficient mice. Transl Psychiatry 3, e280 (2013).
Brynskikh, A., Warren, T., Zhu, J. & Kipnis, J. Adaptive immunity affects learning behavior in mice. Brain Behav. Immun. 22, 861–869 (2008).
Ron-Harel, N. et al. Age-dependent spatial memory loss can be partially restored by immune activation. Rejuven. Res. 11, 903–913 (2008).
Radjavi, A., Smirnov, I. & Kipnis, J. Brain antigen-reactive CD4+ T cells are sufficient to support learning behavior in mice with limited T cell repertoire. Brain Behav. Immun. 35, 58–63 (2014).
Derecki, N. C., Quinnies, K. M. & Kipnis, J. Alternatively activated myeloid (M2) cells enhance cognitive function in immune compromised mice. Brain Behav. Immun. 25, 379–385 (2011).
Baudouin, S. J. Heterogeneity and convergence: the synaptic pathophysiology of autism. Eur. J. Neurosci. 39, 1107–1113 (2014).
Patterson, P. H. Modeling autistic features in animals. Pediat. Res. 69, 34R–40R (2011).
Giovanoli, S. et al. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science 339, 1095–1099 (2013). A proof-of-concept study showing that accretion of environmental risk factors leads to distinct neurodevelopmental pathophysiology.
Abbas, A. K., Lichtman, A. H. & Pillai, S. Cellular and Molecular Immunology 7th edn (Saunders, 2012).
Schmitz, M. L., Weber, A., Roxlau, T., Gaestel, M. & Kracht, M. Signal integration, crosstalk mechanisms and networks in the function of inflammatory cytokines. Biochim. Biophys. Acta 1813, 2165–2175 (2011).
Cavaillon, J. M. Pro- versus anti-inflammatory cytokines: myth or reality. Cell. Mol. Biol. 47, 695–702 (2001).
Yirmiya, R. & Goshen, I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 25, 181–213 (2011).
Schwartz, M. & Shechter, R. Protective autoimmunity functions by intracranial immunosurveillance to support the mind: the missing link between health and disease. Mol. Psychiatry 15, 342–354 (2010).
Xanthos, D. N. & Sandkuhler, J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat. Rev. Neurosci. 15, 43–53 (2014). An excellent opinion piece cautioning against an 'inflammatory-centric' perspective of immune changes in the brain.
Wang, J. et al. Wild-type microglia do not reverse pathology in mouse models of Rett syndrome. Nature 521, E1–E4 (2015).
Acknowledgements
The authors thank members of the McAllister laboratory for ongoing discussions about the topics covered in this Review, especially B. M. Elmer. M.L.E. has been supported by a Dennis Weatherstone Predoctoral Fellowship from Autism Speaks (#7825), the Letty and James Callinan and Cathy and Andrew Moley Fellowship from the ARCS (Achievement Rewards for College Scientists) Foundation, and a Dissertation Year Fellowship from the University of California Office of the President. A.K.M. is supported by grants from the National Institute of Neurological Disorders and Stroke (NINDS; R01-NS060125-05), the National Institute of Mental Health (NIMH; P50-MH106438-01), the Simons Foundation (SFARI #321998), and the University of California Davis Research Investments in Science and Engineering Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Autoimmune disorders
-
Disorders wherein the immune system attacks normal substances and tissues of the body.
- Maternal immune activation
-
(MIA). An animal model of prenatal immune challenge generated by stimulating the maternal immune system with viral or bacterial mimics, live antigens or inflammatory cytokines.
- Copy number variants
-
Deletions or duplications of chromosomal segments that lead to phenotypic diversity among individuals.
- Single-nucleotide polymorphism
-
(SNP). The most common form of genetic variation due to nucleotide substitutions.
- Human leukocyte antigen
-
(HLA). The gene locus that encodes the human versions of three different classes of major histocompatibility complex proteins.
- Complement
-
A system of plasma proteins that attack extracellular pathogens, assist in pathogen and cellular debris clearance by phagocytes and facilitate synaptic pruning in the brain.
- Maternal immunoglobulin G (IgG) antibodies
-
IgG antibodies that pass through the placenta during the third trimester and enter fetal circulation, where they persist at high titre levels for several months after birth.
- Blood–brain barrier
-
(BBB). A selectively permeable network of endothelial cells, pericytes and astrocytes separating the circulating blood from the brain extracellular fluid. The BBB begins to form in the first trimester and is fully formed by birth in humans. Infection, disease and certain drugs can increase the permeability of the BBB.
- Fetal-brain-reactive antibodies
-
Maternally derived immunoglobulin G antibodies that can cross the placenta and bind to fetal brain proteins.
- Polyinosinic–polycytidylic acid
-
(Poly(I:C)). Mismatched double-stranded RNA that acts as a viral mimic.
- Gut microbiota
-
A diverse set of microorganisms that inhabit the gut and shape host immune function.
- Phagocytosis
-
The engulfment of extracellular pathogens or cellular debris by certain immune cells, including microglia.
Rights and permissions
About this article
Cite this article
Estes, M., McAllister, A. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat Rev Neurosci 16, 469–486 (2015). https://doi.org/10.1038/nrn3978
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3978
This article is cited by
-
Maternal immune activation alters fetal and neonatal microglia phenotype and disrupts neurogenesis in mice
Pediatric Research (2023)
-
Maternal immune activation affects socio-communicative behavior in adult rats
Scientific Reports (2023)
-
Fetal inflammatory response and risk for psychiatric disorders
Translational Psychiatry (2023)
-
Immunoregulatory and/or Anti-inflammatory Agents for the Management of Core and Associated Symptoms in Individuals with Autism Spectrum Disorder: A Narrative Review of Randomized, Placebo-Controlled Trials
CNS Drugs (2023)
-
Brief Report: A Double-Blind, Placebo-Controlled, Crossover, Proof-of-Concept Study of Minocycline in Autism Spectrum Disorder
Journal of Autism and Developmental Disorders (2023)