Key Points
-
Liver failure may affect cerebral function, leading to hepatic encephalopathy (HE), a neuropsychiatric condition that may present different forms and grades of severity. Liver failure may be acute or chronic (for example, cirrhosis), and each condition induces different neurological alterations.
-
Acute liver failure (ALF) may lead to rapid coma and death. Many patients with ALF die of intracranial hypertension and brain herniation. Ammonia and inflammation are main contributors to these alterations.
-
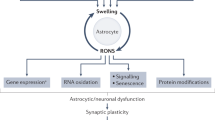
In rats with ALF, there is an initial disruption of the blood–brain barrier, leading to vasogenic oedema in some brain areas (for example, the cerebellum). Brain ammonia and glutamine levels progressively increase, leading later to cytotoxic oedema in many areas. NMDA receptor activation, increased lactate and altered cerebral blood flow are alterations that occur later and also contribute to the increase in intracranial pressure. Blockade of NMDA receptors delays death in rats with ALF.
-
Chronic liver disease (for example, cirrhosis) leads to progressive alterations in sleep, and cognitive and motor function. HE in cirrhosis may be clinical or minimal (MHE). In clinical HE, the symptoms are evident, and its severity is graded according to the level of impairment of autonomy, changes in consciousness, intellectual function and behaviour.
-
Between 33 and 50% of cirrhotic patients without evident symptoms of HE show MHE, which is characterized by mild cognitive impairment, attention deficits, psychomotor slowing and visuomotor and bimanual coordination impairment, which can be detected by psychometric tests.
-
Early diagnosis and treatment of MHE would improve the quality of life and lifespan of patients and prevent or delay the progression of neurological impairment. Determination of critical flicker frequency or of serum 3-nitrotyrosine levels may hold promise as new tools for the early diagnosis of MHE.
-
Hyperammonaemia and inflammation have synergistic roles in inducing neurological alterations in MHE.
-
The function of neuronal circuits between the basal ganglia, thalamus and cortex that modulate motor activity are altered in MHE owing to altered dopaminergic, glutamatergic and GABAergic neurotransmission. Hypokinesia in rats with MHE is due to increased extracellular glutamate and activation of metabotropic glutamate receptor 1 (mGluR1) in the substantia nigra pars reticulata (SNr). Blockade of mGluR1 in the SNr normalizes neurotransmission and motor activity. Anti-inflammatory drugs or inhibitors of mitogen-activated protein kinase (MAPK) p38 also restore motor activity.
-
A main contributor to cognitive impairment in MHE is the reduced function of the glutamate–nitric oxide–cyclic GMP pathway in the cerebellum, resulting in reduced formation of cGMP in response to activation of NMDA receptors. Learning ability in a Y maze task can be restored in rats with MHE by increasing cGMP in the cerebellum.
-
Learning ability may be restored in rats with MHE by using inhibitors of phosphodiesterase 5, anti-inflammatory drugs (ibuprofen), inhibitors of MAPK p38 or modulators of GABAA receptors, which restore the function of the glutamate–nitric oxide–cGMP pathway and extracellular cGMP. Translation of these results to clinical practice would help to improve cognitive function in patients with MHE.
Abstract
Liver failure affects brain function, leading to neurological and psychiatric alterations; such alterations are referred to as hepatic encephalopathy (HE). Early diagnosis of minimal HE reveals an unexpectedly high incidence of mild cognitive impairment and psychomotor slowing in patients with liver cirrhosis — conditions that have serious health, social and economic consequences. The mechanisms responsible for the neurological alterations in HE are beginning to emerge. New therapeutic strategies acting on specific targets in the brain (phosphodiesterase 5, type A GABA receptors, cyclooxygenase and mitogen-activated protein kinase p38) have been shown to restore cognitive and motor function in animal models of chronic HE, and NMDA receptor antagonists have been shown to increase survival in acute liver failure. This article reviews the latest studies aimed at understanding how liver failure affects brain function and potential ways to ameliorate these effects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Stepanova, M., Mishra, A., Venkatesan, C. & Younossi, Z. M. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin. Gastroenterol. Hepatol. 10, 1034–1041 (2012).
Conn, H. O. et al. Comparison of lactulose and neomycin in the treatment of chronic portal-systemic encephalopathy. A double blind controlled trial. Gastroenterology 72, 573–583 (1977).
Córdoba, J. New assessment of hepatic encephalopathy. J. Hepatol. 54, 1030–1040 (2011).
Ferenci, P. et al. Hepatic encephalopathy. Definition, nomenclature, diagnosis and quantification: final report of the working party at the 11th World Congress of Gastroenterology, Vienna, 1998. Hepatology 35, 716–721 (2002).
Kircheis, G., Wettstein, M., Timmermann, L., Schnitzler, A. & Häussinger, D. Critical flicker frequency for quantification of low-grade hepatic encephalopathy. Hepatology 35, 357–366 (2002).
Montoliu, C. et al. 3-nitro-tyrosine as a peripheral biomarker of the presence of minimal hepatic encephalopathy in patients with liver cirrhosis. Am. J. Gastroenterol. 106, 1629–1637 (2011).
Kale, R. A. et al. Demonstration of interstitial cerebral edema with diffusion tensor MR imaging in type C HE. Hepatology 43, 698–706 (2006).
Jover, R. et al. Brain edema and inflammatory activation in bile duct ligated rats and diet-induced hyperammonemia: a model of hepatic encephalopathy in cirrhosis. Hepatology 43, 1257–1266 (2006).
Oria, M. et al. Motor-evoked potentials in awake rats are a valid method of assessing hepatic encephalopathy and of studying its pathogenesis. Hepatology 52, 2077–2085 (2010).
Cauli, O. et al. Cerebral edema is not responsible for motor or cognitive deficits in rats with hepatic encephalopathy. Liver Int. http://dx.doi.org/10.1111/liv.12258 (2013).
Tofteng, F. et al. Persistent arterial hyperammonemia increases the concentration of glutamine and alanine in the brain and correlates with intracranial pressure in patients with fulminant hepatic failure. J. Cereb. Blood Flow Metab. 26, 21–27 (2006).
Hermenegildo, C. et al. NMDA receptor antagonists prevent acute ammonia toxicity in mice. Neurochem. Res. 21, 1237–1244 (1996).
Cauli, O. et al. Brain region selective mechanisms contribute to the progression of cerebral alterations in acute liver failure in rats. Gastroenterology 140, 638–645 (2011).
Jalan, R., Olde Damink, S. W., Hayes, P. C., Deutz, N. E. & Lee, A. Pathogenesis of intracranial hypertension in acute liver failure: inflammation, ammonia and cerebral blood flow. J. Hepatol. 41, 613–620 (2004).
Jiang, W., Desjardins, P. & Butterworth, R. F. Cerebral inflammation contributes to encephalopathy and brain edema in acute liver failure: protective effect of minocycline. J. Neurochem. 109, 485–493 (2009).
Cauli, O. et al. Acute liver failure-induced death of rats is delayed or prevented by blocking NMDA receptors in brain. Am. J. Physiol. Gastr. Liv Physiol. 295, G503–G511 (2008).
Shawcross, D.L., Davies, N.A., Williams, R. & Jalan, R. Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J. Hepatol. 40, 247–254 (2004).
Montoliu, C. et al. IL-6 and IL-18 in blood may discriminate cirrhotic patients with and without minimal hepatic encephalopathy. J. Clin. Gastroenterol. 43, 272–279 (2009).
Cauli, O. et al. Neuroinflammation contributes to hypokinesia in rats with hepatic encephalopathy. Ibuprofen restores its motor activity. J. Neurosci. Res. 87, 1369–1374 (2009).
Zemtsova, I. et al. Microglia activation in hepatic encephalopathy in rats and humans. Hepatology 54, 204–215 (2011).
Cauli, O., Rodrigo, R., Piedrafita, B., Boix, J. & Felipo, V. Inflammation and hepatic encephalopathy: ibuprofen restores learning ability in rats with porto-caval shunts. Hepatology 46, 514–519 (2007). This article shows that rats with MHE have neuroinflammation and that anti-inflammatory drugs restore the function of the glutamate–nitric oxide–cGMP pathway and learning ability.
Rodrigo, R. et al. Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology 139, 675–684 (2010). This article shows that chronic hyperammonaemia per se induces microglial activation and neuroinflammation, which mediates learning impairments and hypokinesia in rats with HE.
Marini, J. C. & Broussard, S. R. Hyperammonemia increases sensitivity to LPS. Mol. Genet. Metab. 88, 131–137 (2006).
Felipo, V. et al. Contribution of hyperammonemia and inflammatory factors to cognitive impairment in minimal hepatic encephalopathy. Metab. Brain Dis. 27, 51–58 (2012).
Häussinger, D., Kircheis, G., Fischer, R., Schliess, F. & vom Dahl, S. Hepatic encephalopathy in chronic liver disease: a clinical manifestation of astrocyte swelling and low-grade cerebral edema? J. Hepatol. 32, 1035–1038 (2000).
Córdoba, J. et al. The development of low-grade cerebral edema in cirrhosis is supported by the evolution of 1H-magnetic resonance abnormalities after liver transplantation. J. Hepatol. 35, 598–604 (2001).
Timmermann, L. et al. Mini-asterixis in hepatic encephalopathy induced by pathologic thalamo-motor-cortical coupling. Neurology 61, 689–692 (2003).
Felipo, V. et al. Patients with minimal hepatic encephalopathy show impaired mismatch negativity correlating with reduced performance in attention tests. Hepatology 55, 530–539 (2012).
Zhang, L. J. et al. Altered brain functional connectivity in patients with cirrhosis and minimal hepatic encephalopathy: a functional MR imaging study. Radiology 265, 528–536 (2012).
Qi, R. et al. Structural and functional abnormalities of default mode network in minimal hepatic encephalopathy: a study combining DTI and fMRI. PLoS ONE 7, e41376 (2012). This article shows that patients with MHE have altered functional connectivity in the default-mode network in some regions without structural alterations, indicating that functional alterations precede structural alterations.
Monfort, P., Muñoz, M. D., ElAyadi, A., Kosenko, E. & Felipo, V. Effects of hyperammonemia and liver disease on glutamatergic neurotransmission. Metab. Brain Dis. 17, 237–250 (2002).
Cauli, O. et al. Glutamatergic and gabaergic neurotransmission and neuronal circuits in hepatic encephalopathy. Metab. Brain Dis. 24, 69–80 (2009).
Cauli, O., Mansouri, M. T., Agusti, A. & Felipo, V. Hyperammonemia increases GABAergic tone in cerebellum but decreases it in rat cortex. Gastroenterology 136, 1359–1367 (2009).
Cauli, O., Mlili, N., Llansola, M. & Felipo, V. Motor activity is modulated via different neuronal circuits in rats with chronic liver failure than in normal rats. Eur. J. Neurosci. 25, 2112–2122 (2007).
Cauli, O., Llansola, M., Erceg, S. & Felipo, V. Hypolocomotion in rats with chronic liver failure is due to increased glutamate and activation of metabotropic glutamate receptors in substantia nigra. J. Hepatol. 45, 654–661 (2006).
Agusti, A. et al. p38 MAP kinase is a therapeutic target for hepatic encephalopathy in rats with portacaval shunts. Gut 60, 1572–1579 (2011).
Monfort, P., Erceg, S., Piedrafita, B., Llansola, M. & Felipo, V. Chronic liver failure in rats impairs glutamatergic synaptic transmission and long-term potentiation in hippocampus and learning ability. Eur. J. Neurosci. 25, 2103–2111 (2007).
Erceg, S. et al. Oral administration of sildenafil restores learning ability in rats with hyperammonemia and with portacaval shunt. Hepatology 45, 2–10 (2005). This article identifies a mechanism that leads to reduced learning ability in rats with MHE and provides evidence of a treatment that restores learning.
Corbalán, R., Chatauret, N., Behrends, S., Butterworth, R. F. & Felipo, V. Region selective alterations of soluble guanylate cyclase content and modulation in brain of cirrhotic patients. Hepatology 36, 1155–1162 (2002).
El-Mlili, N., Rodrigo, R., Naghizadeh, B., Cauli, O. & Felipo, V. Chronic hyperammonemia reduces the activity of neuronal nitric oxide synthase in cerebellum by altering its localization and increasing its phosphorylation by calcium-calmodulin kinase II. J. Neurochem. 106, 1440–1449 (2008).
Vaquero, J. Therapeutic hypothermia in the management of acute liver failure. Neurochem. Int. 60, 723–735 (2012).
Jalan, R. et al. Moderate hypothermia in patients with acute liver failure and uncontrolled intracranial hypertension. Gastroenterology 127, 1338–1346 (2004).
Bass, N. M. et al. Rifaximin treatment in hepatic encephalopathy. N. Engl. J. Med. 362, 1071–1081 (2010).
Bajaj, J. S. et al. Rifaximin improves driving simulator performance in a randomized trial of patients with minimal hepatic encephalopathy. Gastroenterology. 140, 478–487 (2011).
Mittal, V. V., Sharma, B. C., Sharma, P. & Sarin, S. K. A randomized controlled trial comparing lactulose, probiotics, and l-ornithine l-aspartate in treatment of minimal hepatic encephalopathy. Eur. J. Gastroenterol. Hepatol. 23, 725–732 (2011).
Wang, Y. W., Lin, H. C., Yang, Y. Y., Hou, M. C. & Lee, S. D. Sildenafil decreased pulmonary arterial pressure but may have exacerbated portal hypertension in a patient with cirrhosis and portopulmonary hypertension. J. Gastroenterol. 41, 593–597 (2006).
Butterworth, R. F. et al. Experimental models of hepatic encephalopathy: ISHEN guidelines. Liver Int 29, 783–788 (2009).
Acknowledgements
This work was supported by grants from the Ministerio Economia y Competitividad Spain (SAF2011-23051; CSD2008-00005) and the Consellería Educación generalitat Valenciana, (PROMETEO-2009-027; ACOMP/2013/101).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Intracranial pressure
-
The pressure inside the cranium (brain and cerebrospinal fluid).
- Psychomotor slowing
-
Slowing of physical and psychological reactions, such as movement or speech, owing to impairment of mental processes.
- Psychometric Hepatic Encephalopathy Score
-
(PHES). A battery of five psychometric tests: digit symbol test (DST), number connection test A (NCT-A), number connection test B (NCT-B), serial dotting test (SD) and line-tracing test (LTT). PHES is currently considered the gold standard for assessing minimal hepatic encephalopathy in patients with cirrhosis.
- Cytotoxic oedema
-
Accumulation of water inside cells; this leads to astrocyte swelling.
- Systemic inflammatory response syndrome
-
(SIRS). An inflammatory state affecting the whole body that is defined by the coexistence of two or more of these conditions: fever or hypothermia, tachycardia, tachypnea and an abnormally high leukocyte count. It can be of either infectious or non-infectious in origin.
- Vasogenic oedema
-
Accumulation of extracellular water between the body's cells (interstitial spaces). This tends to occur when the blood–brain barrier is permeabilized.
- Functional MRI
-
(fMRI). A technique for the detection and delineation of brain regions that change their level of activation in response to specific experimental conditions. It can also detect resting-state networks.
- Apparent diffusion coefficient
-
A parameter determined by magnetic resonance that measures the magnitude of diffusion of water molecules within cerebral tissue.
- Mismatch negativity
-
(MMN). An auditory-evoked potential that occurs after an infrequent change in a repetitive sequence of sounds. MMN is assessed by electroencephalography and reflects neuronal activity related to pre-attentional processes.
- Default-mode network
-
A network of brain regions that are active when an individual is awake and at rest.
- Hypokinesia
-
Abnormally diminished motor function or activity. It is usually associated with basal ganglia diseases.
- Portopulmonary hypertension
-
The coexistence of portal and pulmonary hypertension. It is a serious complication of liver cirrhosis.
Rights and permissions
About this article
Cite this article
Felipo, V. Hepatic encephalopathy: effects of liver failure on brain function. Nat Rev Neurosci 14, 851–858 (2013). https://doi.org/10.1038/nrn3587
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3587
This article is cited by
-
Extracellular vesicles from mesenchymal stem cells reduce neuroinflammation in hippocampus and restore cognitive function in hyperammonemic rats
Journal of Neuroinflammation (2023)
-
Effects of oxidative stress on hepatic encephalopathy pathogenesis in mice
Nature Communications (2023)
-
Heart rate variability is associated with encephalopathy and outcomes in pediatric acute liver failure
Pediatric Research (2023)
-
J-difference GABA-edited MRS reveals altered cerebello-thalamo-cortical metabolism in patients with hepatic encephalopathy
Metabolic Brain Disease (2023)
-
Sustained Hyperammonemia Activates NF-κB in Purkinje Neurons Through Activation of the TrkB-PI3K-AKT Pathway by Microglia-Derived BDNF in a Rat Model of Minimal Hepatic Encephalopathy
Molecular Neurobiology (2023)