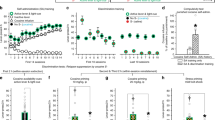
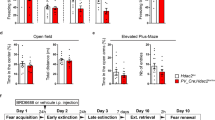
Abstract
Correlational data suggest that learned associations are encoded within neuronal ensembles. However, it has been difficult to prove that neuronal ensembles mediate learned behaviours because traditional pharmacological and lesion methods, and even newer cell type-specific methods, affect both activated and non-activated neurons. In addition, previous studies on synaptic and molecular alterations induced by learning did not distinguish between behaviourally activated and non-activated neurons. Here, we describe three new approaches — Daun02 inactivation, FACS sorting of activated neurons and Fos-GFP transgenic rats — that have been used to selectively target and study activated neuronal ensembles in models of conditioned drug effects and relapse. We also describe two new tools — Fos-tTA transgenic mice and inactivation of CREB-overexpressing neurons — that have been used to study the role of neuronal ensembles in conditioned fear.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hebb, D. O. The Organization of Behavior: a Neuropsychological Theory (Wiley, 1949).
Schwindel, C. D. & McNaughton, B. L. Hippocampal–cortical interactions and the dynamics of memory trace reactivation. Prog. Brain Res. 193, 163–177 (2011).
Nicolelis, M. A., Fanselow, E. E. & Ghazanfar, A. A. Hebb's dream: the resurgence of cell assemblies. Neuron 19, 219–221 (1997).
Guzowski, J. F., Knierim, J. J. & Moser, E. I. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron 44, 581–584 (2004).
Pennartz, C. M., Groenewegen, H. J. & Lopes da Silva, F. H. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog. Neurobiol. 42, 719–761 (1994).
Knierim, J. J. & Zhang, K. Attractor dynamics of spatially correlated neural activity in the limbic system. Annu. Rev. Neurosci. 35, 267–285 (2012).
Buzsaki, G. & Moser, E. I. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nature Neurosci. 16, 130–138 (2013).
Penner, M. R. & Mizumori, S. J. Neural systems analysis of decision making during goal-directed navigation. Prog. Neurobiol. 96, 96–135 (2012).
Mountcastle, V. B. Modality and topographic properties of single neurons of cat's somatic sensory cortex. J. Neurophysiol. 20, 408–434 (1957).
John, E. R. & Schwartz, E. L. The neurophysiology of information processing and cognition. Annu. Rev. Psychol. 29, 1–29 (1978).
O'Keefe, J. A review of the hippocampal place cells. Prog. Neurobiol. 13, 419–439 (1979).
Carelli, R. M. & Deadwyler, S. A. Cellular mechanisms underlying reinforcement-related processing in the nucleus accumbens: electrophysiological studies in behaving animals. Pharmacol. Biochem. Behav. 57, 495–504 (1997).
Maren, S. Neurobiology of Pavlovian fear conditioning. Annu. Rev. Neurosci. 24, 897–931 (2001).
Guzowski, J. F., McNaughton, B. L., Barnes, C. A. & Worley, P. F. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nature Neurosci. 2, 1120–1124 (1999).
Mattson, B. J. et al. Context-specific sensitization of cocaine-induced locomotor activity and associated neuronal ensembles in rat nucleus accumbens. Eur. J. Neurosci. 27, 202–212 (2008).
Reijmers, L. G., Perkins, B. L., Matsuo, N. & Mayford, M. Localization of a stable neural correlate of associative memory. Science 317, 1230–1233 (2007).
Guzowski, J. F. et al. Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr. Opin. Neurobiol. 15, 599–606 (2005).
Garner, A. & Mayford, M. New approaches to neural circuits in behavior. Learn. Mem. 19, 385–390 (2012).
Liu, X. et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (2012).
Grienberger, C. & Konnerth, A. Imaging calcium in neurons. Neuron 73, 862–885 (2012).
Harvey, C. D., Collman, F., Dombeck, D. A. & Tank, D. W. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature 461, 941–946 (2009).
Ziv, Y. et al. Long-term dynamics of CA1 hippocampal place codes. Nature Neurosci. 16, 264–266 (2013).
Rogan, S. C. & Roth, B. L. Remote control of neuronal signaling. Pharmacol. Rev. 63, 291–315 (2011).
Sweatt, J. D. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J. Neurochem. 76, 1–10 (2001).
Alberini, C. M. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 28, 51–56 (2005).
Tronson, N. C. & Taylor, J. R. Molecular mechanisms of memory reconsolidation. Nature Rev. Neurosci. 8, 262–275 (2007).
Yizhar, O., Fenno, L. E., Davidson, T. J., Mogri, M. & Deisseroth, K. Optogenetics in neural systems. Neuron 71, 9–34 (2011).
Koya, E. et al. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nature Neurosci. 12, 1069–1073 (2009).
Guez-Barber, D. et al. FACS identifies unique cocaine-induced gene regulation in selectively activated adult striatal neurons. J. Neurosci. 31, 4251–4259 (2011).
Cifani, C. et al. Medial prefrontal cortex neuronal activation and synaptic alterations after stress-induced reinstatement of palatable food seeking: a study using c-fos-GFP transgenic female rats. J. Neurosci. 32, 8480–8490 (2012).
Han, J. H. et al. Selective erasure of a fear memory. Science 323, 1492–1496 (2009).
Zhou, Y. et al. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nature Neurosci. 12, 1438–1443 (2009).
Garner, A. R. et al. Generation of a synthetic memory trace. Science 335, 1513–1516 (2012).
Morgan, J. I. & Curran, T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu. Rev. Neurosci. 14, 421–451 (1991).
Cohen, S. & Greenberg, M. E. Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu. Rev. Cell Dev. Biol. 24, 183–209 (2008).
Herdegen, T. & Leah, J. D. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res. Brain Res. Rev. 28, 370–490 (1998).
Goldberg, S. R. Stimuli associated with drug injections as events that control behavior. Pharmacol. Rev. 27, 325–340 (1976).
Stewart, J., de Wit, H. & Eikelboom, R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol. Rev. 91, 251–268 (1984).
O'Brien, C. P., Ehrman, R. N. & Ternes, J. W. in Behavioral Analysis of Drug Dependence (eds Goldberg, S. & Stolerman, I.) 329–356 (Academic Press, 1986).
Wikler, A. Dynamics of drug dependence. Implications of a conditioning theory for research and treatment. Arch. Gen. Psychiatry 28, 611–616 (1973).
Siegel, S. Drug anticipation and drug addiction. The 1998 H. David Archibald Lecture. Addiction 94, 1113–1124 (1999).
Carelli, R. M. The nucleus accumbens and reward: neurophysiological investigations in behaving animals. Behav. Cogn. Neurosci. Rev. 1, 281–296 (2002).
Rebec, G. V. & Sun, W. Neuronal substrates of relapse to cocaine-seeking behavior: role of prefrontal cortex. J. Exp. Anal. Behav. 84, 653–666 (2005).
Schuster, C. R. & Thompson, T. Self administration of and behavioral dependence on drugs. Annu. Rev. Pharmacol. 9, 483–502 (1969).
Chang, J. Y., Zhang, L., Janak, P. H. & Woodward, D. J. Neuronal responses in prefrontal cortex and nucleus accumbens during heroin self-administration in freely moving rats. Brain Res. 754, 12–20 (1997).
Kiyatkin, E. A. & Rebec, G. V. Activity of presumed dopamine neurons in the ventral tegmental area during heroin self-administration. Neuroreport 8, 2581–2585 (1997).
Carelli, R. M., King, V. C., Hampson, R. E. & Deadwyler, S. A. Firing patterns of nucleus accumbens neurons during cocaine self-administration in rats. Brain Res. 626, 14–22 (1993).
Peoples, L. L. & West, M. O. Phasic firing of single neurons in the rat nucleus accumbens correlated with the timing of intravenous cocaine self-administration. J. Neurosci. 16, 3459–3473 (1996).
Carelli, R. M., Williams, J. G. & Hollander, J. A. Basolateral amygdala neurons encode cocaine self-administration and cocaine-associated cues. J. Neurosci. 23, 8204–8211 (2003).
Root, D. H., Fabbricatore, A. T., Ma, S., Barker, D. J. & West, M. O. Rapid phasic activity of ventral pallidal neurons during cocaine self-administration. Synapse 64, 704–713 (2010).
Bowers, M. S., Chen, B. T. & Bonci, A. AMPA receptor synaptic plasticity induced by psychostimulants: the past, present, and therapeutic future. Neuron 67, 11–24 (2010).
Russo, S. J. et al. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 33, 267–276 (2010).
Wolf, M. E. & Ferrario, C. R. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci. Biobehav. Rev. 35, 185–211 (2010).
Thomas, M. J., Kalivas, P. W. & Shaham, Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br. J. Pharmacol. 154, 327–342 (2008).
Kalivas, P. W. & Volkow, N. D. The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatry 162, 1403–1413 (2005).
Shaham, Y. & Hope, B. T. The role of neuroadaptations in relapse to drug seeking. Nature Neurosci. 8, 1437–1439 (2005).
Mameli, M. & Luscher, C. Synaptic plasticity and addiction: learning mechanisms gone awry. Neuropharmacology 61, 1052–1059 (2011).
Shaham, Y., Shalev, U., Lu, L., De Wit, H. & Stewart, J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology 168, 3–20 (2003).
Marchant, N. J., Li, X. & Shaham, Y. Recent developments in animal models of drug relapse. Curr. Opin. Neurobiol. 23, 675–683 (2013).
Pickens, C. L. et al. Neurobiology of incubation of drug craving. Trends Neurosci. 34, 411–420 (2011).
Kasof, G. M. et al. Spontaneous and evoked glutamate signalling influences Fos-lacZ expression and pyramidal cell death in hippocampal slice cultures from transgenic rats. Brain Res. Mol. Brain Res. 34, 197–208 (1995).
Kasof, G. M. et al. Kainic acid-induced neuronal death is associated with DNA damage and a unique immediate-early gene response in c-fos–lacZ transgenic rats. J. Neurosci. 15, 4238–4249 (1995).
Kasof, G. M., Smeyne, R. J., Curran, T. & Morgan, J. I. Developmental expression of Fos-lacZ in the brains of postnatal transgenic rats. Brain Res. Dev. Brain Res. 93, 191–197 (1996).
Bakina, E. & Farquhar, D. Intensely cytotoxic anthracycline prodrugs: galactosides. Anticancer Drug Des. 14, 507–515 (1999).
Farquhar, D. et al. Suicide gene therapy using E. coli β-galactosidase. Cancer Chemother. Pharmacol. 50, 65–70 (2002).
Ghosh, A. K., Khan, S., Marini, J., Nelson, J. C. & Farquhar, D. A daunorubicin β-galactoside prodrug for use in conjunction with gene-directed enzyme prodrug therapy. Tetrahedron Lett. 41, 4871–4874 (2000).
Bossert, J. M. et al. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nature Neurosci. 14, 420–422 (2011).
Fanous, S. et al. Role of orbitofrontal cortex neuronal ensembles in the expression of incubation of heroin craving. J. Neurosci. 32, 11600–11609 (2012).
Santone, K. S., Oakes, S. G., Taylor, S. R. & Powis, G. Anthracycline-induced inhibition of a calcium action potential in differentiated murine neuroblastoma cells. Cancer Res. 46, 2659–2664 (1986).
Badiani, A. & Robinson, T. E. Drug-induced neurobehavioral plasticity: the role of environmental context. Behav. Pharmacol. 15, 327–339 (2004).
Stewart, J. & Badiani, A. Tolerance and sensitization to the behavioral effects of drugs. Behav. Pharmacol. 4, 289–312 (1993).
Mullen, R. J., Buck, C. R. & Smith, A. M. NeuN, a neuronal specific nuclear protein in vertebrates. Development 116, 201–211 (1992).
Crombag, H., Bossert, J. M., Koya, E. & Shaham, Y. Context-induced relapse to drug seeking: a review. Trans. R. Soc. B 363, 3233–3243 (2008).
Grimm, J. W., Hope, B. T., Wise, R. A. & Shaham, Y. Incubation of cocaine craving after withdrawal. Nature 412, 141–142 (2001).
Shalev, U., Morales, M., Hope, B., Yap, J. & Shaham, Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology 156, 98–107 (2001).
Neisewander, J. L. et al. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J. Neurosci. 20, 798–805 (2000).
Lobo, M. K. Molecular profiling of striatonigral and striatopallidal medium spiny neurons past, present, and future. Int. Rev. Neurobiol. 89, 1–35 (2009).
Lobo, M. K., Karsten, S. L., Gray, M., Geschwind, D. H. & Yang, X. W. FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nature Neurosci. 9, 443–452 (2006).
Dougherty, J. D. & Geschwind, D. H. Progress in realizing the promise of microarrays in systems neurobiology. Neuron 45, 183–185 (2005).
Karsten, S. L., Kudo, L. C. & Geschwind, D. H. Gene expression analysis of neural cells and tissues using DNA microarrays. Curr. Protoc. Neurosci. 45, 4.28.1–4.28.38 (2008).
Guez-Barber, D. et al. FACS purification of immunolabeled cell types from adult rat brain. J. Neurosci. Methods 203, 10–18 (2012).
Fanous, S. et al. Unique gene alterations are induced in FACS-purified Fos-positive neurons activated during cue-induced relapse to heroin seeking. J. Neurochem. 124, 100–108 (2013).
Everitt, B. J. & Robbins, T. W. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neurosci. 8, 1481–1489 (2005).
Kelley, A. E. & Berridge, K. C. The neuroscience of natural rewards: relevance to addictive drugs. J. Neurosci. 22, 3306–3311 (2002).
Ikemoto, S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens–olfactory tubercle complex. Brain Res. Rev. 56, 27–78 (2007).
Liu, Q. R. et al. Detection of molecular alterations in methamphetamine-activated Fos-expressing neurons from a single rat dorsal striatum using fluorescence-activated cell sorting (FACS). J. Neurochem. http://dx.doi.org/10.1111/jnc.12381 (2013).
Bredt, D. S. & Nicoll, R. A. AMPA receptor trafficking at excitatory synapses. Neuron 40, 361–379 (2003).
Martin, S. J., Grimwood, P. D. & Morris, R. G. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu. Rev. Neurosci. 23, 649–711 (2000).
Luscher, C. & Malenka, R. C. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69, 650–663 (2011).
Benedetti, B. L., Takashima, Y., Wen, J. A., Urban-Ciecko, J. & Barth, A. L. Differential wiring of layer 2/3 neurons drives sparse and reliable firing during neocortical development. Cereb.Cortex http://dx.doi.org/10.1093/cercor/bhs257 (2012).
Clem, R. L. & Barth, A. Pathway-specific trafficking of native AMPARs by in vivo experience. Neuron 49, 663–670 (2006).
Barth, A. L. Visualizing circuits and systems using transgenic reporters of neural activity. Curr. Opin. Neurobiol. 17, 567–571 (2007).
Barth, A. L., Gerkin, R. C. & Dean, K. L. Alteration of neuronal firing properties after in vivo experience in a FosGFP transgenic mouse. J. Neurosci. 24, 6466–6475 (2004).
Yassin, L. et al. An embedded subnetwork of highly active neurons in the neocortex. Neuron 68, 1043–1050 (2010).
Koya, E. et al. Silent synapses in selectively activated nucleus accumbens neurons following cocaine sensitization. Nature Neurosci. 15, 1556–1562 (2012).
Isaac, J. T., Crair, M. C., Nicoll, R. A. & Malenka, R. C. Silent synapses during development of thalamocortical inputs. Neuron 18, 269–280 (1997).
Brown, T. E. et al. A silent synapse-based mechanism for cocaine-induced locomotor sensitization. J. Neurosci. 31, 8163–8174 (2011).
Huang, Y. H. et al. In vivo cocaine experience generates silent synapses. Neuron 63, 40–47 (2009).
Nair, S. G., Adams-Deutsch, T., Epstein, D. H. & Shaham, Y. The neuropharmacology of relapse to food seeking: methodology, main findings, and comparison with relapse to drug seeking. Prog. Neurobiol. 89, 18–45 (2009).
Ghitza, U. E., Gray, S. M., Epstein, D. H., Rice, K. C. & Shaham, Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF1 receptors. Neuropsychopharmacology 31, 2188–2196 (2006).
Nair, S. G. et al. Role of dorsal medial prefrontal cortex dopamine D1-family receptors in relapse to high-fat food seeking induced by the anxiogenic drug yohimbine. Neuropsychopharmacology 36, 497–510 (2011).
Calu, D. J. et al. Optogenetic inhibition of dorsal medial prefrontal cortex attenuates stress-induced reinstatement of palatable food seeking in female rats. J. Neurosci. 33, 214–226 (2013).
Bremner, J. D., Krystal, J. H., Southwick, S. M. & Charney, D. S. Noradrenergic mechanisms in stress and anxiety: II. clinical studies. Synapse 23, 39–51 (1996).
Ungless, M. A., Whistler, J. L., Malenka, R. C. & Bonci, A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411, 583–587 (2001).
Mennerick, S. & Zorumski, C. F. Paired-pulse modulation of fast excitatory synaptic currents in microcultures of rat hippocampal neurons. J. Physiol. 488, 85–101 (1995).
Quirk, G. J. & Mueller, D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33, 56–72 (2008).
Pape, H. C. & Pare, D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol. Rev. 90, 419–463 (2010).
Paz, R. & Pare, D. Physiological basis for emotional modulation of memory circuits by the amygdala. Curr. Opin. Neurobiol. 23, 381–386 (2013).
Barot, S. K., Chung, A., Kim, J. J. & Bernstein, I. L. Functional imaging of stimulus convergence in amygdalar neurons during Pavlovian fear conditioning. PLoS ONE 4, e6156 (2009).
Chung, A., Barot, S. K., Kim, J. J. & Bernstein, I. L. Biologically predisposed learning and selective associations in amygdalar neurons. Learn. Mem. 18, 371–374 (2011).
Hashikawa, K., Matsuki, N. & Nomura, H. Preferential Arc transcription at rest in the active ensemble during associative learning. Neurobiol. Learn. Mem. 95, 498–504 (2011).
Purgert, R. J., Wheeler, D. S., McDannald, M. A. & Holland, P. C. Role of amygdala central nucleus in aversive learning produced by shock or by unexpected omission of food. J. Neurosci. 32, 2461–2472 (2012).
Josselyn, S. A. Continuing the search for the engram: examining the mechanism of fear memories. J. Psychiatry Neurosci. 35, 221–228 (2010).
Reijmers, L. & Mayford, M. Genetic control of active neural circuits. Front. Mol. Neurosci. 2, 27 (2009).
Alexander, G. M. et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63, 27–39 (2009).
Tayler, K. K., Tanaka, K. Z., Reijmers, L. G. & Wiltgen, B. J. Reactivation of neural ensembles during the retrieval of recent and remote memory. Curr. Biol. 23, 99–106 (2013).
Han, J. H. et al. Neuronal competition and selection during memory formation. Science 316, 457–460 (2007).
Dong, Y. et al. CREB modulates excitability of nucleus accumbens neurons. Nature Neurosci. 9, 475–477 (2006).
Marie, H., Morishita, W., Yu, X., Calakos, N. & Malenka, R. C. Generation of silent synapses by acute in vivo expression of CaMKIV and CREB. Neuron 45, 741–752 (2005).
Guenthner, C. J., Miyamichi, K., Yang, H. H., Heller, H. C. & Luo, L. Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron 78, 773–784 (2013).
Peter, M. et al. Transgenic mouse models enabling photolabeling of individual neurons in vivo. PLoS ONE 8, e62132 (2013).
Sanders, J., Cowansage, K., Baumgartel, K. & Mayford, M. Elimination of dendritic spines with long-term memory is specific to active circuits. J. Neurosci. 32, 12570–12578 (2012).
Matsuo, N., Reijmers, L. & Mayford, M. Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. Science 319, 1104–1107 (2008).
Cao, V. Y. et al. In vivo two-photon imaging of experience-dependent molecular changes in cortical neurons. J. Vis. Exp. 71, e50148 (2013).
Kawashima, T. et al. Synaptic activity-responsive element in the Arc/Arg-3.1 promoter essential for synapse-to-nucleus signaling in activated neurons. Proc. Natl Acad. Sci. USA 106, 316–321 (2009).
Johnson, J. W. & Kotermanski, S. E. Mechanism of action of memantine. Curr. Opin. Pharmacol. 6, 61–67 (2006).
Sinner, B. & Graf, B. M. Ketamine. Handb. Exp. Pharmacol. 182, 313–333 (2008).
Wood, P. L. The NMDA receptor complex: a long and winding road to therapeutics. IDrugs 8, 229–235 (2005).
Nader, K., Schafe, G. E. & LeDoux, J. E. The labile nature of consolidation theory. Nature Rev. Neurosci. 1, 216–219 (2000).
Lerea, L. S., Butler, L. S. & McNamara, J. O. NMDA and non-NMDA receptor-mediated increase of c-fos mRNA in dentate gyrus neurons involves calcium influx via different routes. J. Neurosci. 12, 2973–2981 (1992).
Sgambato, V., Abo, V., Rogard, M., Besson, M. J. & Deniau, J. M. Effect of electrical stimulation of the cerebral cortex on the expression of the Fos protein in the basal ganglia. Neuroscience 81, 93–112 (1997).
Vazdarjanova, A. & Guzowski, J. F. Differences in hippocampal neuronal population responses to modifications of an environmental context: evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. J. Neurosci. 24, 6489–6496 (2004).
Hope, B. T. et al. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron 13, 1235–1244 (1994).
Sheng, M. & Greenberg, M. E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron 4, 477–485 (1990).
Hardingham, G. E., Chawla, S., Johnson, C. M. & Bading, H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature 385, 260–265 (1997).
Valjent, E., Caboche, J. & Vanhoutte, P. Mitogen-activated protein kinase/extracellular signal-regulated kinase induced gene regulation in brain: a molecular substrate for learning and memory? Mol. Neurobiol. 23, 83–99 (2001).
Thomas, G. M. & Huganir, R. L. MAPK cascade signalling and synaptic plasticity. Nature Rev. Neurosci. 5, 173–183 (2004).
Mattson, B. J. et al. Cocaine-induced CREB phosphorylation in nucleus accumbens of cocaine-sensitized rats is enabled by enhanced activation of extracellular signal-related kinase, but not protein kinase A. J. Neurochem. 95, 1481–1494 (2005).
LaHoste, G. J., Yu, J. & Marshall, J. F. Striatal Fos expression is indicative of dopamine D1/D2 synergism and receptor supersensitivity. Proc. Natl Acad. Sci. USA 90, 7451–7455 (1993).
Hardingham, G. E., Arnold, F. J. & Bading, H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nature Neurosci. 4, 261–267 (2001).
Deisseroth, K., Mermelstein, P. G., Xia, H. & Tsien, R. W. Signaling from synapse to nucleus: the logic behind the mechanisms. Curr. Opin. Neurobiol. 13, 354–365 (2003).
O'Donnell, P. Dopamine gating of forebrain neural ensembles. Eur. J. Neurosci. 17, 429–435 (2003).
Labiner, D. M. et al. Induction of c-fos mRNA by kindled seizures: complex relationship with neuronal burst firing. J. Neurosci. 13, 744–751 (1993).
Kreuter, J. D., Mattson, B. J., Wang, B., You, Z. B. & Hope, B. T. Cocaine-induced Fos expression in rat striatum is blocked by chloral hydrate or urethane. Neuroscience 127, 233–242 (2004).
Acknowledgements
The writing of this article was supported by the US National Institute on Drug Abuse, Intramural Research Program. We thank the members of the Hope, Lupica and Shaham laboratories who contributed to the development and implementation of the new technologies described in this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Cruz, F., Koya, E., Guez-Barber, D. et al. New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat Rev Neurosci 14, 743–754 (2013). https://doi.org/10.1038/nrn3597
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3597
This article is cited by
-
Alcohol-specific transcriptional dynamics of memory reconsolidation and relapse
Translational Psychiatry (2023)
-
Cocaine-induced projection-specific and cell type-specific adaptations in the nucleus accumbens
Molecular Psychiatry (2022)
-
Addiction as a brain disease revised: why it still matters, and the need for consilience
Neuropsychopharmacology (2021)
-
Improving translation of animal models of addiction and relapse by reverse translation
Nature Reviews Neuroscience (2020)
-
Silent synapses dictate cocaine memory destabilization and reconsolidation
Nature Neuroscience (2020)