Key Points
-
Recent neurobiological insights into this gut–brain crosstalk have revealed a complex, bidirectional communication system that not only assures proper maintenance of gastrointestinal homeostasis and digestion but is likely to have multiple effects on affect, motivation and higher cognitive functions.
-
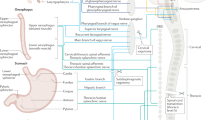
Sympathetic and parasympathetic innervations modulate intestinal function and are likely to mediate the reported emotion-related patterns of regional changes in motor, secretory and possibly immune activity in the gastrointestinal tract.
-
There are three basic mechanisms by which sensory information is encoded in the gut: by primary afferent neurons, by immune cells and by enteroendocrine cells.
-
Both extrinsic and intrinsic primary afferents provide input to multiple reflex loops that are aimed at optimizing gut function and maintaining gastrointestinal homeostasis during internal perturbations.
-
The output of enteroendocrine cells is involved both in the regulation of digestive functions through enteric nervous system circuits, as well as in the regulation of CNS processes through endocrine and paracrine signalling to vagal afferents.
-
Immune cells in the gut remain immunologically hyporesponsive to commensal bacteria, while maintaining their responsiveness to pathogenic organisms, and their products indirectly influence the functional properties of enteroendocrine cells.
-
Recent evidence suggests that various forms of subliminal interoceptive inputs from the gut, including those generated by intestinal microbes, may influence memory formation, emotional arousal and affective behaviours. The human insula, and related brain networks (including the anterior cingulate cortex, orbitofrontal cortex and amygdala), has emerged as the most plausible brain region to support this integration.
-
It remains to be determined whether intuitive decision making is based on an interoceptive map of gut responses that enables the brain to make rapid gut-based decisions based on interoceptive memories of such responses.
-
There is extensive evidence of alterations in brain–gut signalling systems during perturbation to gut homeostasis, in several chronic gastrointestinal disorders and in eating disorders. Further understanding of the bidirectional crosstalk between the brain and the digestive system may aid the development of effective therapies for these conditions.
Abstract
The concept that the gut and the brain are closely connected, and that this interaction plays an important part not only in gastrointestinal function but also in certain feeling states and in intuitive decision making, is deeply rooted in our language. Recent neurobiological insights into this gut–brain crosstalk have revealed a complex, bidirectional communication system that not only ensures the proper maintenance of gastrointestinal homeostasis and digestion but is likely to have multiple effects on affect, motivation and higher cognitive functions, including intuitive decision making. Moreover, disturbances of this system have been implicated in a wide range of disorders, including functional and inflammatory gastrointestinal disorders, obesity and eating disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Furness, J. B. The Enteric Nervous System (Blackwell, Oxford, 2006). A comprehensive overview of all aspects of the enteric nervous system.
Furness, J. B., Kunze, W. A., Bertrand, P. P., Clerc, N. & Bornstein, J. C. Intrinsic primary afferent neurons of the intestine. Prog. Neurobiol. 54, 1–18 (1998).
Costa, M., Furness, J. B., Llewellyn-Smith, I. J. & Johnson, L. R. in Physiology of the Gastrointestinal Tract (eds Johnson, L. R. et al.) 1–40 (Raven, New York, 1987).
Gershon, M. D. et al. in Physiology of the Gastrointestinal Tract (eds Johnson, L. R. et al.) 381–422 (Raven Press, New York, 1994).
Gershon, M. D. The Second Brain (Harper Collins, New York, 1998).
Mayer, E. A. & Brunnhuber, S. in Handbook of Clinical Neurology 3rd edn (in the press).
Cannon, W. B. Organization for physiological homeostasis. Physiol. Rev. 9, 399–431 (1929).
James, W. What is an emotion? Mind 9, 188–205 (1884).
Mayer, E. A. et al. Brain imaging approaches to the study of functional GI disorders: a Rome working team report. Neurogastroenterol. Motil. 21, 579–596 (2009).
Round, J. L. & Mazmanian, S. K. The gut microbiota shapes intestinal immune responses during health and disease. Nature Rev. Immunol. 9, 313–323 (2009).
Artis, D. & Grencis, R. K. The intestinal epithelium: sensors to effectors in nematode infection. Mucosal Immunol. 1, 252–264 (2008).
Rhee, S. H., Pothoulakis, C. & Mayer, E. A. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nature Rev. Gastroenterol. Hepatol. 6, 306–314 (2009). A review of emerging concepts and preclinical evidence that support the idea of intestinal microbiota to brain signalling.
Collins, S. M. & Bercik, P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology 136, 2003–2014 (2009).
Forsythe, P., Sudo, N., Dinan, T., Taylor, V. H. & Bienenstock, J. Mood and gut feelings. Brain Behav. Immun. 24, 9–16 (2010).
Price, J. L., Carmichael, S. T. & Drevets, W. C. Networks related to the orbital and medial prefrontal cortex: a substrate for emotional behavior? Prog. Brain Res. 107, 523–536 (1996).
Ongur, D. & Price, J. L. The organization of networks within the orbital and medical prefrontal cortex of rats, monkeys and humans. Cereb. Cortex 10, 206–219 (2000).
Vogt, B. A. in The Cerebral Cortex (eds Jones, E. G. & Peters, A.) 89–149 (Plenum Press, New York, 1985).
Bandler, R., Keay, K. A. in The Emotional Motor System. Progress in Brain Research (eds Holstege, G., Bandler, R. & Saper, C. B.) 285–300 (Elsevier, Amsterdam, 1996).
Holstege, G. et al. in The Emotional Motor System (eds Jones, E. G. & Peters, A.) 3–6 (Elsevier, Amsterdam, 1996).
Mayer, E. A. The neurobiology of stress and gastrointestinal disease. Gut 47, 861–869 (2000).
Mason, P. From descending pain modulation to obesity via the medullary raphe. Pain 152, S20–S24 (2010).
Fields, H. State-dependent opioid control of pain. Nature Rev. Neurosci. 5, 565–575 (2004).
Martinez, V. & Taché, Y. CRF1 receptors as a therapeutic target for irritable bowel syndrome. Curr. Pharm. Des. 12, 4071–4088 (2006).
Almy, T. P., Kern, F. & Tulin, M. Alterations in colonic function in man under stress. I: experimental production of sigmoid spasm in healthy persons. Gastroenterology 8, 616–626 (1947).
Welgan, P., Meshkinpour, H. & Ma, L. Role of anger in antral motor activity in irritable bowel syndrome. Dig. Dis. Sci. 45, 248–251 (2000).
Valentino, R. J., Miselis, R. R. & Pavcovich, L. A. Pontine regulation of pelvic viscera: pharmacological target for pelvic visceral dysfunction. Trends in Pharmacol. Sci. 20, 253–260 (1999).
Browning, K. N. & Travagli, R. A. Plasticity of vagal brainstem circuits in the control of gastrointestinal function. Auton. Neurosci. 161, 6–13 (2011).
Jaenig, W. in The Integrative Action of the Autonomic Nervous System (ed. Jaenig, W.) 317–318 (Cambridge Univ. Press, New York, 2006).
Furness, J. B. & Costa, M. The adrenergic innervation of the gastrointestinal tract. Ergeb. Physiol. 69, 1–51 (1974).
Elenkov, I. J., Wilder, R. L., Chrousos, G. P. & Vizi, E. S. The sympathetic nerve - an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 52, 585–638 (2000).
Lyte, M., Vulchanova, L. & Brown, D. R. Stress at the intestinal surface: catecholamines and mucosa-bacteria interactions. Cell Tissue Res. 343, 23–32 (2010). A comprehensive overview of evidence for interactions between peripheral stress mediators and bidirectional interactions of the mucosa and intestinal microbiota.
Hori, T., Katafuchi, T., Take, S., Shimizu, N. & Niijima, A. The autonomic nervous system as a communication channel between the brain and the immune system. Neuroimmunomodulation 2, 203–215 (1995).
Gopal, R., Birdsell, D. & Monroy, F. P. Regulation of toll-like receptors in intestinal epithelial cells by stress and Toxoplasma gondii infection. Parasite Immunol. 30, 563–576 (2008).
Powley, T. L. et al. in Brain–Gut Interactions (eds Tache, Y. & Wingate, D.) 73–82 (CRC Press, Boston, 1991).
Stephens, R. L. & Tache, Y. Intracisternal injection of a TRH analogue stimulates gastric luminal serotonin release in rats. Am. J. Physiol. 256, G377–G383 (1989).
Pavlov, V. A. & Tracey, K. J. The cholinergic anti-inflammatory pathway. Brain Behav. Immun. 19, 493–499 (2005).
Almy, T. P., Kern, F. & Tulin, M. Alterations in colonic function in man under stress. II: experimental production of sigmoid spasm in healthy persons. Gastroenterology 12, 425–436 (1949).
Welgan, P., Meshkinpour, H. & Beeler, M. Effect of anger on colon motor and myoelectric activity in irritable bowel syndrome. Gastroenterology 94, 1150–1156 (1988).
Elenkov, I. J. & Chrousos, G. P. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann. NY Acad. Sci. 966, 290–303 (2002).
Khasar, S. G. et al. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J. Neurosci. 28, 5721–5730 (2008). The first evidence that psychosocial stressors can modulate the phenotype of afferent neurons, providing the possible mechanisms for stress induced hyperalgesia.
Seminowicz, D. A. et al. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology 139, 48–57 (2010).
Wood, J. D. in Physiology of the Gastrointestinal Tract (ed. Johnson, L. R.) 67–110 (Raven, New York, 1987).
Kurokawa, K. et al. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res. 14, 169–181 (2007).
Clerc, N. & Furness, J. B. Intrinsic primary afferent neurones of the digestive tract. Neurogastroenterol. Motil. 16, 24–27 (2004).
Sengupta, J. N. & Gebhart, G. F. in Physiology of the Gastrointestinal Tract (ed. Johnson, L. R.) 483–519 (Raven, New York, 1994).
Keita, A. V. & Soderholm, J. D. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol. Motil. 22, 718–733 (2010).
Kunze, W. A. & Furness, J. B. The enteric nervous system and regulation of intestinal motility. Annu. |Rev. Physiol. 61, 117–142 (1999).
Grundy, D. et al. Fundamentals of neurogastroenterology: basic science. Gastroenterology 130, 1391–1411 (2006).
Phillips, R. J. & Powley, T. L. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res. Brain Res. Rev. 34, 1–26 (2000).
Lynn, P., Zagorodnyuk, V., Hennig, G., Costa, M. & Brookes, S. Mechanical activation of rectal intraganglionic laminar endings in the guinea pig distal gut. J. Physiol. 564, 589–601 (2005).
Sanders, K. M. Interstitial cells in smooth muscles. Review series. J. Cell. Mol. Med. 14, 1197–1198 (2010).
Brierley, S. M. Molecular basis of mechanosensitivity. Auton. Neurosci. 153, 58–68 (2009).
Kyloh, M. Nicholas, S., Zagorodnyuk, V. P., Brookes, S. J. & Spencer, N. J. Identification of the visceral pain pathway activated by noxious colorectal distension in mice. Front. Neurosci. 5, 16 (2011).
Grundy, D. Signalling the state of the digestive tract. Auton. Neurosci. 125, 76–80 (2006).
Brierley, S. M., Jones, R. C., Gebhart, G. F. & Blackshaw, L. A. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127, 166–178 (2004).
Gold, M. S. & Gebhart, G. F. Nociceptor sensitization in pain pathogenesis. Nature Med. 16, 1248–1257 (2010).
Feng, B. & Gebhart, G. F. Characterization of silent afferents in the pelvic and splanchnic innervations of the mouse colorectum. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G170–G180 (2010).
Gershon, M. D. Nerves, reflexes, and the enteric nervous system: pathogenesis of the irritable bowel syndrome. J. Clin. Gastroenterol. 39, S184–S193 (2005).
de Lartigue, G., de La Serre, C. B. & Raybould, H. E. Vagal afferent neurons in high fat diet-induced obesity; intestinal microflora, gut inflammation and cholecystokinin. Physiol. Behav. 2 Mar 2011 (doi:10.1016/j.physbeh.2011.02.040). A review of interactions between intestinal microflora, mucosal inflammation and altered cholecystokinin–vagal interactions in high fat induced obesity.
Dockray, G. J. Cholecystokinin and gut-brain signalling. Regul. Pept. 155, 6–10 (2009).
de Lartigue, G. et al. Cocaine- and amphetamine-regulated transcript mediates the actions of cholecystokinin on rat vagal afferent neurons. Gastroenterology 138, 1479–1490 (2009).
Barbara, G. et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 132, 26–37 (2007).
Raybould, H. E. Gut chemosensing: interactions between gut endocrine cells and visceral afferents. Auton. Neurosci. 153, 41–46 (2009).
Gershon, M. D. & Tack, J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132, 397–414 (2007). A comprehensive review of the role of the gut-based serotonin signalling system in health and its possible role in gastrointestinal disease.
McLaughlin, J. T. et al. Fatty acids stimulate cholecystokinin secretion via an acyl chain length-specific, Ca2+-dependent mechanism in the enteroendocrine cell line STC-1. J. Physiol. 513, 11–18 (1998).
Liou, A. P. et al. The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology 140, 903–912 (2011).
Rozengurt, E. & Sternini, C. Taste receptor signaling in the mammalian gut. Curr. Opin. Pharmacol. 7, 557–562 (2007).
Hughes, D. T. & Sperandio, V. Inter-kingdom signalling: communication between bacteria and their hosts. Nature Rev. Microbiol. 6, 111–120 (2008).
Leslie, F. C. et al. Plasma cholecystokinin concentrations are elevated in acute upper gastrointestinal infections. QJM 96, 870–871 (2003).
McDermott, J. R. et al. Immune control of food intake: enteroendocrine cells are regulated by CD4+ T lymphocytes during small intestinal inflammation. Gut 55, 492–497 (2006).
Rozengurt, E. Taste receptors in the gastrointestinal tract. I. Bitter taste receptors and α-gustducin in the mammalian gut. Am. J. Physiol. Gastrointest. Liver Physiol. 291, G171–G177 (2006).
Artis, D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nature Rev. Immunol. 8, 411–420 (2008).
Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 449, 819–826 (2007).
Mawe, G. M., Strong, D. S. & Sharkey, K. A. Plasticity of enteric nerve functions in the inflamed and postinflamed gut. Neurogastroenterol. Motil. 21, 481–491 (2009).
Berntson, G. G., Sarter, M. & Cacioppo, J. T. Ascending visceral regulation of cortical affective information processing. Eur. J. Neurosci. 18, 2103–2109 (2003).
Craig, A. D. An ascending general homeostatic afferent pathway originating in lamina I. Prog. Brain Res. 107, 225–242 (1996).
Craig, A. D. Interoception: the sense of the physiological condition of the body. Curr. Opin. Neurobiol. 13, 500–505 (2003).
Craig, A. D. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Rev. Neurosci. 3, 655–666 (2002).
Craig, A. in Handbook of Emotions 3rd edn (eds Lewis, M. & Haviland-Jones, J. M.) 272–288 (Guilford Publications, New York, 2008).
Craig, A. D. How do you feel-now? The anterior insula and human awareness. Nature Rev. Neurosci. 10, 59–70 (2009).
Damasio, A. R. The Feeling of What Happens: Body and Emotion in the Making of Consciousness (Harcourt Brace, New York, New York, 1999).
Kurth, F., Zilles, K., Fox, P. T., Laird, A. R. & Eickhoff, S. B. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct. Funct. 214, 519–534 (2010). A comprehensive quantitative meta-analysis of published neuroimaging studies involving insula activation, providing the best evidence to date for function-specific subregions of the human insula.
Mutschler, I. et al. Functional organization of the human anterior insular cortex. Neurosci. Lett. 457, 66–70 (2009).
Penfield, W. & Faulk, M. E. Jr. The insula; further observations on its function. Brain 78, 445–470 (1955).
Augustine, J. R. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res. Rev. 22, 229–244 (1996).
Cauda, F. et al. Functional connectivity of the insula in the resting brain. Neuroimage 55, 8–23 (2011). A detailed characterization of resting state connectivity of insula subregions, supporting the concept of function-specific networks related to ventral and dorsal subregions of the anterior insular cortex.
Small, D. M. & Prescott, J. Odor/taste integration and the perception of flavor. Exp. Brain Res. 166, 345–357 (2005).
Small, D. M. Central gustatory processing in humans. Adv. Otorhinolaryngol. 63, 191–220 (2006).
Kaye, W. H., Fudge, J. L. & Paulus, M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nature Rev. Neurosci. 10, 573–584 (2009). A comprehensive review of current biological concepts related to eating disorders, with an emphasis on altered processing and modulation of intereoceptive signals.
Bercik, P. The microbiota-gut-brain axis: learning from intestinal bacteria? Gut 60, 288–289 (2011).
Bercik, P. et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology 139, 2102–2112.e1 (2010). The first demonstration of an important role of intestinal inflammation in brain signalling systems and associated behavioural changes in rodents.
Heijtz, R. D. et al. Normal gut microbiota modulates brain development and behavior. Proc. Natl Acad. Sci. USA 108, 3047–3052 (2011). The first demonstration of an important role of intestinal microbiota early in life in brain development and adult anxiety-like behaviour.
Sclafani, A. Oral and postoral determinants of food reward. Physiol. Behav. 81, 773–779 (2004).
Van Oudenhove, L. et al. Emotional modulation of fatty acid induced gut-brain signalling in brainstem, subcortical and cortical regions: an fMRI study. Gastroenterology 138, S-45 (2010). The first demonstration in humans of an interaction between subliminal gut stimuli and experimentally induced emotional states.
Berman, S. M. et al. Abnormal CNS response to anticipation of visceral distension in female patients with irritable bowel syndrome (IBS) an FMRI study. Gastroenterology 130, A-78 (2006).
Ploghaus, A., Becerra, L., Borras, C. & Borsook, D. Neural circuitry underlying pain modulation: expectation, hypnosis, placebo. Trends Cogn. Sci. 7, 197–200 (2003).
Wicker, B. et al. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron 40, 655–664 (2003).
Grill, H. J. & Norgren, R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 143, 263–279 (1978).
Steiner, J. E. The gustofacial response: observation on normal and anencephalic newborn infants. Symp. Oral Sens. Percept. 254–278 (1973).
Foo, H. & Mason, P. Analgesia accompanying food consumption requires ingestion of hedonic foods. J. Neurosci. 29, 13053–13062 (2009).
Pepino, M. Y. & Mennella, J. A. Sucrose-induced analgesia is related to sweet preferences in children but not adults. Pain 119, 210–218 (2005).
Jiang, H., Betancourt, L. & Smith, R. G. Ghrelin amplifies dopamine signaling by cross talk involving formation of growth hormone secretagogue receptor/dopamine receptor subtype 1 heterodimers. Mol. Endocrinol. 20, 1772–1785 (2006).
Preuschoff, K., Quartz, S. R. & Bossaerts, P. Human insula activation reflects risk prediction errors as well as risk. J. Neurosci. 28, 2745–2752 (2008). An elegant demonstration of the involvement of ventral and dorsal anterior insula subregions in risk prediction and error correction, supporting the concept of function-specific insula subregions.
Ploghaus, A. et al. Learning about pain: the neural substrate of the prediction error for aversive events. Proc. Natl Acad. Sci. USA 97, 9281–9286 (2000).
Bechara, A., Damasio, A. R., Damasio, H. & Anderson, S. W. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50, 7–15 (1994).
Allman, J. M. et al. The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct. Funct. 214, 495–517 (2010).
Gershon, M. D. Review article: serotonin receptors and transporters - roles in normal and abnormal gastrointestinal motility. Aliment. Pharmacol. Ther. 20, 3–14 (2004).
Watkins, L. R. & Maier, S. F. The pain of being sick: implications of immune-to-brain communication for understanding pain. Annu. Rev. Psychol. 51, 29–57 (2000).
la Fleur, S. E., Wick, E. C., Idumalla, P. S., Grady, E. F. & Bhargava, A. Role of peripheral corticotropin-releasing factor and urocortin II in intestinal inflammation and motility in terminal ileum. Proc. Natl Acad. Sci. USA 102, 7647–7652 (2005).
Maier, S. F. & Watkins, L. R. Cytokines for psychologists: implications and bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol. Rev. 105, 83–107 (1998).
Singer, T., Critchley, H. D. & Preuschoff, K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn. Sci. 13, 334–340 (2009).
Vergnolle, N. Postinflammatory visceral sensitivity and pain mechanisms. Neurogastroenterol. Motil. 20, 73–80 (2008).
Watkins, L. R. & Maier, S. F. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J. Intern. Med. 257, 139–155 (2005).
Dunn, A. J. Mechanisms by which cytokines signal the brain. Int. Rev. Neurobiol. 52, 43–65 (2002).
D'Mello, C., Le, T. & Swain, M. G. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factorα signaling during peripheral organ inflammation. J. Neurosci. 29, 2089–2102 (2009).
Holzer, P. Efferent-like roles of afferent neurons in the gut: blood flow regulation and tissue protection. Auton. Neurosci. 125, 70–75 (2006).
Berthoud, H. R. & Morrison, C. The brain, appetite, and obesity. Annu. Rev. Psychol. 59, 55–92 (2008).
Drossman, D. A. et al. (eds) ROME III: The Functional Gastrointestinal Disorders (Degnon Associates, McLean, Virginia 2006).
Longstreth, G. F. et al. in ROME III: The Functional Gastrointestinal Disorders (eds Drossman, D. A. et al.) 487–556 (Degnon Associates, McLean, Virginia, 2006).
Mayer, E. A. Clinical practice. Irritable bowel syndrome. N. Engl. J. Med. 358, 1692–1699 (2008).
Mayer, E. A. & Tillisch, K. The brain-gut axis in abdominal pain syndromes. Annu. Rev. Med. 62, 381–396 (2011).
Tillisch, K. & Labus, J. S. Advances in imaging the brain-gut axis: functional gastrointestinal disorders. Gastroenterology 140, 407–411e1 (2011).
Mayer, E. A. et al. Functional GI disorders: from animal models to drug development. Gut 57, 384–404 (2008).
Blankstein, U., Chen, J., Diamant, N. E. & Davis, K. D. Altered brain structure in irritable bowel syndrome: potential contributions of pre-existing and disease-driven factors. Gastroenterology 138, 1783–1789 (2010).
Mayer, E. A. & Bushnell, M. C. in Functional Pain Syndromes: Presentation and Pathophysiology (eds Mayer, E. A. & Bushnell, M. C.) 531–565 (IASP Press, Seattle, 2009).
Sengupta, J. N. Visceral pain: the neurophysiological mechanism. Handb. Exp. Pharmacol. 194, 31–74 (2009).
Spiller, R. C. Postinfectious irritable bowel syndrome. Gastroenterology 124, 1662–1671 (2003).
Verma-Gandhu, M. et al. Visceral pain perception is determined by the duration of colitis and associated neuropeptide expression in the mouse. Gut 56, 358–364 (2007).
Chang, L. et al. Differences in brain responses to rectal distension between patients with inflammatory and functional GI disorders. Gastroenterology 126, A-106 (2004).
Graff, L. A., Walker, J. R. & Bernstein, C. N. Depression and anxiety in inflammatory bowel disease: a review of comorbidity and management. Inflamm. Bowel Dis. 15, 1105–1118 (2009).
Tache, Y. & Bernstein, C. N. Evidence for the role of the brain-gut axis in inflammatory bowel disease: depression as cause and effect? Gastroenterology 136, 2058–2061 (2009).
Ghia, J. E. et al. Reactivation of inflammatory bowel disease in a mouse model of depression. Gastroenterology 136, 2280–2288.e1–4 (2009). An interesting study demonstrating the interaction of stress, emotional states and gut inflammation.
Fairburn, C. G. & Harrison, P. J. Eating disorders. Lancet 361, 407–416 (2003).
Finucane, M. M. et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 377, 557–567 (2011).
Volkow, N. D., Wang, G. J. & Baler, R. D. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn. Sci. 15, 37–46 (2011). An excellent review of data and concepts related to food addiction and obesity.
Padwal, R. et al. Bariatric surgery: a systematic review and network meta-analysis of randomized trials. Obes. Rev. 28 Mar 2011 (doi:10.1111/j.1467-789X.2011.00866.x).
Paulino, G. et al. Increased expression of receptors for orexigenic factors in nodose ganglion of diet-induced obese rats. Am. J. Physiol. Endocrinol. Metab. 296, e898–e903 (2009). An interesting study that demonstrates the molecular mechanisms underlying fat induced phenotypic changes in vagal afferents, which may play a part in the decreased influences of interoceptive mechanisms in diet-induced obesity.
Paulus, M. P. & Stein, M. B. An insular view of anxiety. Biol. Psychiatry 60, 383–387 (2006).
Grupe, D. W. & Nitschke, J. B. Uncertainty is associated with biased expectancies and heightened responses to aversion. Emotion 11, 413–424 (2011).
Sarinopoulos, I. et al. Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cereb. Cortex 20, 929–940 (2010).
Sutton, R. S. & Barton, A. G. Reinforcement Learning: An Introduction (MIT press, Cambridge, USA, 1998).
Behrens, T. E., Woolrich, M. W., Walton, M. E. & Rushworth, M. F. Learning the value of information in an uncertain world. Nature Neurosci. 10, 1214–1221 (2007). An elegant study demonstrating how human subjects assess uncertainty and adjust their decision making accordingly. The study indentifies a key role of brain responses of the anterior cingulate cortex in this process.
Ganfornina, M. D., Sanchez, D. & Bastiani, M. J. Embryonic development of the enteric nervous system of the grasshopper Schistocerca americana. J. Comp. Neurol. 372, 581–596 (1996).
Campbell, G. & Burnstock, G. in Handbook of Physiology: the Gastrointestinal System (ed. Code, C. F.) 2213–2266 (American Physiological Society, Washington D. C., 1968).
Shimizu, H., Koizumi, O. & Fujisawa, T. Three digestive movements in Hydra regulated by the diffuse nerve net in the body column. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 190, 623–630 (2004).
Bullock, T. H. & Horridge, G. A. Structure and function in the nervous system of invertebrates (W. H. Freeman & Co., San Francisco and London, 1965).
Gershon, M. D., Chalazonitis, A. & Rothman, T. P. From neural crest to bowel: development of the enteric nervous system. J. Neurobiol. 24, 199–214 (1993).
MacLean, P. D., Vogt, B. A. & Gabriel, M. in Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook 1–15 (Birkhäuser, Boston, 1993).
Damasio, A. R. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Phil. Trans. R. Soc. Lond. B 351, 1413–1420 (1996).
Acknowledgements
Supported by grants DK048351, DK064539, DK082370 and AT002681 from the National Institutes of Health.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Glossary
- Enteric nervous system
-
Ganglionated plexus of neurons that is located between the layers of the gut. These neurons, which are equal in number to those in the spinal cord, are able to regulate basic gut functions, such as the peristaltic reflex.
- Emotional motor system
-
System of parallel outflows from cortico–limbic–pontine networks that is engaged during distinct homeostatic states. A medial component provides tonic modulation of spinal reflexes and a lateral component plays a part in executing distinct regional motor patterns of the viscera through autonomic pathways
- Enteroendocrine cell
-
Specialized epithelial cell that releases secretory granules, containing one or several gut peptides, on the basolateral side (and possibly luminal side) in response to luminal chemical, mechanical and possibly neural stimuli.
- Enterochromaffin cell
-
Specialized epithelial cell that releases secretory granules, containing primarily serotonin, on the basolateral side (and possibly luminal side) in response to luminal chemical, mechanical and possibly neural stimuli.
- Intrinsic reflex
-
Also known as an intramural reflex. A reflex of the enteric nervous system in which the afferents, interneuron and efferent neurons that are involved are all contained within the gut wall.
- Intrinsic, primary afferent
-
Afferent neuron with its cell body contained within the enteric nervous system, that encodes mechanical and paracrine signals.
- Commensal bacteria
-
Refers to the 100 trillion bacteria that live in symbiosis with the gut and make up the intestinal microflora.
- Myenteric
-
Subplexus of the enteric nervous system, which is localized between the circular and longitudinal muscle layer.
- Ego-syntonic
-
Psychological term referring to behaviours, values and feelings that are in harmony with, or acceptable to, the needs and goal of a person or that are consistent with a person's ideal self image. This trait is typically seen in patients with anorexia nervosa.
Rights and permissions
About this article
Cite this article
Mayer, E. Gut feelings: the emerging biology of gut–brain communication. Nat Rev Neurosci 12, 453–466 (2011). https://doi.org/10.1038/nrn3071
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3071
This article is cited by
-
Symptom effects and central mechanism of acupuncture in patients with functional gastrointestinal disorders: a systematic review based on fMRI studies
BMC Gastroenterology (2024)
-
Trauma, early life stress, and mindfulness in adulthood
BMC Psychology (2024)
-
Migraine and the microbiota. Can probiotics be beneficial in its prevention? - a narrative review
Pharmacological Reports (2024)
-
Gut-Brain-axis: effect of basil oil on the gut microbiota and its contribution to the anticonvulsant properties
BMC Complementary Medicine and Therapies (2023)
-
Host fecal DNA specific methylation signatures mark gut dysbiosis and inflammation in children affected by autism spectrum disorder
Scientific Reports (2023)