Key Points
-
At least three different viewpoints have emerged of cerebellar cortical topography, based on the anatomy and physiology of climbing fibre inputs and Purkinje cell outputs, the physiology of mossy fibre inputs, restriction boundaries in gene expression and regional differences in Purkinje cell phenotype.
-
In this Review we consider some of the more commonly used terminology to describe cerebellar cortical organization with a view to providing some clarification. Overarching this, we argue the different terminologies are in fact unnecessary because both developmental and adult studies suggest that the different mapping techniques — anatomical, physiological and molecular — reveal different facets of a common topography.
-
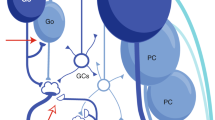
The one-map hypothesis proposes that cerebellar architecture is built around an array of interdigitated transverse zones, each of which is subdivided into a series of rostrocaudally oriented Purkinje cell stripes, defined by the restricted expression of molecular markers, that are symmetrically distributed across the midline, highly reproducible between individuals, and conserved across species. The most comprehensively studied marker is zebrin II, which cloning studies revealed to be the metabolic enzyme aldolase C.
-
The molecular identity of each stripe may already be determined at the time the Purkinje cells become postmitotic, and, at the time a mouse is born, the molecular identity of individual Purkinje cells seems to be set and independent of cerebellar connectivity. The embryonic Purkinje cell clusters are the targets of the developing climbing fibre and mossy fibre afferents and, through this matching, the Purkinje cells form a template around which afferent topography is constructed.
-
Anatomical and physiological studies in adult animals have shown that climbing fibre afferents from different parts of the contralateral inferior olive form longitudinal zones within the cerebellar cortex. In some cases these zones can be subdivided into smaller units called microzones. Investigation of the relationship between longitudinal zones and Purkinje cell stripes has revealed extensive co-localization, consistent with the one-map hypothesis.
-
Anatomical studies have also found that mossy fibre terminal fields align with Purkinje cell stripes and also with climbing fibres associated with individual longitudinal zones, suggesting a common principle of organization.
-
By contrast, other studies support the notion that mossy fibres terminate as patches to form a fractured somatotopical map within the cerebellar cortex. The apparent discrepancy between stripes and patches may be explained by individual Purkinje cell stripes being subdivided into chains of small patches or microzones, which can be revealed by differential gene expression or electrophysiological mapping.
-
The one-map hypothesis therefore proposes that transverse zones are subdivided into stripes (one or more stripes equals a longitudinal zone); and stripes are further segmented longitudinally into microzones that correspond to one or more small patches.
-
The functional significance of this elaborate architecture remains to be determined but may be used for parallel processing of sensorimotor commands, energy efficient information processing, positional coding of sensory inputs and/or molecular fine-tuning of local circuits for specific functions such as motor learning.
Abstract
The fundamental architecture of the cerebellum is concealed within a terminological forest — transverse zones and stripes, longitudinal zones and microzones, patches, etc. To make things worse, the same term is used in different contexts to describe quite different patterns of spatial localization. Here we consider the possibility that this complexity hides the fact that the cerebellar cortex contains only one map, which has been charted in various ways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sillitoe, R. V., Chung, S. H., Fritschy, J. M., Hoy, M. & Hawkes, R. Golgi cell dendrites are restricted by Purkinje cell stripe boundaries in the adult mouse cerebellar cortex. J. Neurosci. 28, 2820–2826 (2008).
Voogd, J. & Bigaré, F. in The Inferior Olivary Nucleus: Anatomy and Physiology (ed. Courville, J.) 207–235 (Raven, New York, 1980).
Apps, R. & Garwicz, M. Anatomical and physiological foundations of cerebellar information processing. Nature Rev. Neurosci. 6, 297–311 (2005).
Larsell, O. The morphogenesis and adult pattern of the lobules and fissures of the cerebellum of the white rat. J. Comp. Neurol. 97, 281–356 (1952).
Larsell, O. The cerebellum of the cat and the monkey. J. Comp. Neurol. 99, 135–199 (1953).
Voogd, J. & Glickstein, M. The anatomy of the cerebellum. Trends Neurosci. 21, 370–375 (1998).
Airey, D. C., Lu, L. & Williams, R. W. Genetic control of the mouse cerebellum: identification of quantitative trait loci modulating size and architecture. J. Neurosci. 21, 5099–5109 (2001).
Larsell, O. The Comparative Anatomy and Histology of the Cerebellum From Monotremes Through Apes (ed. Jansen, J.) (Univ. Minnesota Press, Minneapolis, 1970).
Armstrong, C. L., Krueger-Naug, A. M., Currie, R. W. & Hawkes, R. Constitutive expression of the 25-kDa heat shock protein Hsp25 reveals novel parasagittal bands of Purkinje cells in the adult mouse cerebellar cortex. J. Comp. Neurol. 416, 383–397 (2000).
Beierbach, E., Park, C., Ackerman, S. L., Goldowitz, D. & Hawkes, R. Abnormal dispersion of a Purkinje cell subset in the mouse mutant cerebellar deficient folia (cdf). J. Comp. Neurol. 436, 42–51 (2001).
Howell, B. W., Hawkes, R., Soriano, P. & Cooper, J. A. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature 389, 733–737 (1997).
Trommsdorff, M. et al. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 97, 689–701 (1999).
Larouche, M., Beffert, U., Herz, J. & Hawkes, R. The Reelin receptors Apoer2 and Vldlr coordinate the patterning of Purkinje cell topography in the developing mouse cerebellum. PLoS ONE 3, e1653 (2008).
Atkins, M. J. & Apps, R. Somatotopical organisation within the climbing fibre projection to the paramedian lobule and copula pyramidis of the rat cerebellum. J. Comp. Neurol. 389, 249–263 (1997).
Edge, A. L., Marple-Horvat, D. E. & Apps, R. Lateral cerebellum: functional localization within crus I and correspondence to cortical zones. Eur. J. Neurosci. 18, 1468–1485 (2003).
Thier, P., Dicke, P. W., Haas, R., Thielert, C. D. & Catz, N. The role of the occulomotor vermis in the control of saccadic eye movements. Ann. NY Acad. Sci. 978, 50–62 (2002).
Ozol, K., Hayden, J. M., Oberdick, J. & Hawkes, R. Transverse zones in the vermis of the mouse cerebellum. J. Comp. Neurol. 412, 95–111 (1999).
Korneliussen, H. K. On the ontogenetic development of the cerebellum (nuclei, fissures, and cortex) of the rat, with special reference to regional variations in corticogenesis. J. Hirnforsch. 10, 379–412 (1968).
Sawada, K., Fukui, Y. & Hawkes, R. Spatial distribution of corticotropin-releasing factor immunopositive climbing fibers in the mouse cerebellum: analysis by whole mount immunocytochemistry. Brain Res. 1222, 106–117 (2008).
Marzban, H., Kim, C.-T., Doorn, D., Chung, S.-H. & Hawkes, R. A novel transverse expression domain in the mouse cerebellum revealed by a neurofilament-associated antigen. Neuroscience 153, 721–732 (2008).
Sillitoe, R. V. & Hawkes, R. Whole-mount immunohistochemistry: a high-throughput screen for patterning defects in the mouse cerebellum. J. Histochem. Cytochem. 50, 235–244 (2002).
Brochu, G., Maler, L. & Hawkes, R. Zebrin II: a polypeptide antigen expressed selectively by Purkinje cells reveals compartments in rat and fish cerebellum. J. Comp. Neurol. 291, 538–552 (1990). The first identification of the canonical stripe antigen zebrin II. The zones and stripes revealed by the zebrin II expression map are widely used as a reference frame to correlate maps derived from different experimental techniques.
Ahn, A. H., Dziennis, S., Hawkes, R. & Herrup, K. The cloning of zebrin II reveals its identity with aldolase C. Development 120, 2081–2090 (1994).
Hawkes, R. & Herrup, K. Aldolase C/zebrin II and the regionalization of the cerebellum. J. Mol. Neurosci. 6, 147–158 (1995).
Hawkes, R., Colonnier, M. & Leclerc, N. Monoclonal antibodies reveal sagittal banding in the rodent cerebellar cortex. Brain Res. 333, 359–365 (1985).
Hawkes, R. & Leclerc, N. Antigenic map of the rat cerebellar cortex: the distribution of parasagittal bands as revealed by a monoclonal anti-Purkinje cell antibody mabQ113. J. Comp. Neurol. 256, 29–41 (1987).
Sillitoe, R. V. et al. Conservation of the architecture of the anterior lobe vermis of the cerebellum across mammalian species. Prog. Brain Res. 148, 283–297 (2005).
Scott, T. G. A unique pattern of localization within the cerebellum. Nature 200, 793 (1963). The first demonstration of striped gene expression in the cerebellum — the distribution of 5′-nucleotidase enzyme activity.
Terada, N. et al. Compartmentation of the mouse cerebellar cortex by sphingosine kinase. J. Comp. Neurol. 469, 119–127 (2004).
Jeong, Y. G. et al. The cyclin-dependent kinase 5 activator, p39, is expressed in stripes in the mouse cerebellum. Neuroscience 118, 323–334 (2003).
Sarna, J. R., Marzban, H., Watanabe, M. & Hawkes, R. Complementary stripes of phospholipase Cβ3 and Cβ4 expression by Purkinje cell subsets in the mouse cerebellum. J. Comp. Neurol. 496, 303–313 (2006).
Dehnes, Y. et al. The glutamate transporter EAAT4 in rat cerebellar Purkinje cells: a glutamate-gated chloride channel concentrated near the synapse in parts of the dendritic membrane facing astroglia. J. Neurosci. 18, 3606–3619 (1998).
Mateos, J. M. et al. Parasagittal compartmentalization of the metabotropic glutamate receptor mGluR1b in the cerebellar cortex. Eur. J. Anat. 5, 15–21 (2001).
Murase, S. & Hayashi, Y. Expression pattern of integrin beta 1 subunit in Purkinje cells of rat and cerebellar mutant mice. J. Comp. Neurol. 375, 225–237 (1996).
Chung, S., Zhang, Y., Van der Hoorn, F. & Hawkes, R. The anatomy of the cerebellar nuclei in the normal and scrambler mouse as revealed by the expression of the microtubule-associated protein kinesin light chain 3. Brain Res. 1140, 120–131 (2007).
Marzban, H. et al. Expression of the immunoglobulin superfamily neuroplastin adhesion molecules in adult and developing mouse cerebellum and their localization to parasagittal stripes. J. Comp. Neurol. 462, 286–301 (2003).
Croci, L. et al. A key role for the HLH transcription factor EBF2COE2, O/E-3 in Purkinje neuron migration and cerebellar cortical topography. Development 133, 2719–2729 (2006).
Marzban, H. et al. Abnormal HNK-1 expression in the cerebellum of an N-CAM null mouse. J. Neurocytol. 33, 117–130 (2004).
Marzban, H., Chung, S., Watanabe, M. & Hawkes, R. Phospholipase Cβ4 expression reveals the continuity of cerebellar topography through development. J. Comp. Neurol. 502, 857–871 (2007).
Miale, I. L. & Sidman, R. L. An autoradiographic analysis of histogenesis in the mouse cerebellum. Exp. Neurol. 4, 277–296 (1961).
Armstrong, C. L. & Hawkes, R. Pattern formation in the cerebellar cortex. Biochem. Cell Biol. 78, 551–562 (2000).
Larouche, M., Che, P. E. & Hawkes, R. Neurogranin expression identifies a novel array of Purkinje cell parasagittal stripes during mouse cerebellar development. J. Comp. Neurol. 494, 215–227 (2006).
Sillitoe, R. V. & Joyner, A. L. Morphology, molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annu. Rev. Cell Dev. Biol. 23, 549–577 (2007).
Hashimoto, M. & Mikoshiba, K. Mediolateral compartmentalization of the cerebellum is determined on the “birth date” of Purkinje cells. J. Neurosci. 23, 11342–11351 (2003).
Leclerc, N., Gravel, C. & Hawkes, R. Development of parasagittal zonation in the rat cerebellar cortex. MabQ113 antigenic bands are created postnatally by the suppression of antigen expression in a subset of Purkinje cells. J. Comp. Neurol. 273, 399–420 (1988).
Wassef, M. et al. Expression of compartmentation antigen zebrin I in cerebellar transplants. J. Comp. Neurol. 294, 223–234 (1990).
Seil, F. J., Johnson, M. L. & Hawkes, R. Molecular compartmentation expressed in cerebellar cultures in the absence of neuronal activity and neuron-glia interactions. J. Comp. Neurol. 356, 398–407 (1995).
Oberdick, J. et al. Control of segment-like patterns of gene expression in the mouse cerebellum. Neuron 10, 1007–1018 (1993).
Armstrong, C. L. & Hawkes, R. Selective failure of Purkinje cell dispersion in the cerebellum of the weaver mouse. J. Comp. Neurol. 439, 151–161 (2001).
Gallagher, E., Howell, B. W., Soriano, P., Cooper, J. A. & Hawkes, R. Cerebellar abnormalities in the disabled (mdab1–1) mouse. J. Comp. Neurol. 402, 238–251 (1998).
Goldowitz, D. et al. Cerebellar disorganization characteristic of reeler in scrambler mutant mice despite presence of reelin. J. Neurosci. 17, 8767–8777 (1997).
Edwards, M. A., Crandall, J. E., Leclerc, N. & Yamamoto, M. Effects of nervous mutation on Purkinje cell compartments defined by zebrin II and 9-O-acetylated ganglioside expression. Neurosci. Res. 19, 167–174 (1994).
Sotelo, C. & Wassef, M. Cerebellar development: afferent organization and Purkinje cell heterogeneity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 331, 307–313 (1991).
Wassef, M., Angaut, P., Arsenio Nunes, M. L., Bourrat, F. & Sotelo, C. in The Cerebellum Revisited (ed. Llinás, R. & Sotelo, C.) 5–21 (Springer, New York, 1992).
Sotelo, C. & Chédotal, A. Development of the olivocerebellar system: migration and formation of cerebellar maps. Prog. Brain Res. 148, 1–20 (2005).
Miyata, T., Nakajima, K., Mikoshiba, K. & Ogawa, M. Regulation of Purkinje cell alignment by reelin as revealed with CR-50 antibody. J. Neurosci. 17, 3599–3609 (1997).
Sotelo, C. Cellular and genetic regulation of the development of the cerebellar system. Prog. Neurobiol. 72, 295–339 (2004).
Sotelo, C. & Chédotal, A. Development of the olivocerebellar system: migration and formation of cerebellar maps. Prog. Brain Res. 148, 1–20 (2005).
Karam, S. D. et al. Eph receptors and ephrins in the developing chick cerebellum: relationship to sagittal patterning and granule cell migration. J. Neurosci. 20, 6488–6500 (2000).
Nishida, K., Flanagan, J. G. & Nakamoto, M. Domain-specific olivocerebellar projection regulated by the EphA-ephrin-A interaction. Development 129, 5647–5658 (2002).
Crepel, F. Regression of functional synapses in the immature mammalian cerebellum. Trends Neurosci. 5, 266–269 (1982).
Arsénio Nunes, M. L. & Sotelo, C. Development of the spinocerebellar system in the postnatal rat. J. Comp. Neurol. 237, 291–306 (1985).
Mason, C. A. & Gregory, E. Postnatal maturation of cerebellar mossy and climbing fibers: transient expression of dual features on single axons. J. Neurosci. 4, 1715–1735 (1984).
Takeda, T. & Maekawa, K. Transient direct connection of vestibular mossy fibers to the vestibulocerebellar Purkinje cells in early postnatal development of kittens. Neuroscience 32, 99–111 (1989).
Grishkat, H. L. & Eisenman, L. M. Development of the spinocerebellar projection in the prenatal mouse. J. Comp. Neurol. 363, 93–108 (1995).
Paradies, M. A. et al. Correspondence between L7-lacZ-expressing Purkinje cells and labeled olivocerebellar fibers during late embryogenesis in the mouse. J. Comp. Neurol. 374, 451–466 (1996).
Ji, Z. & Hawkes, R. Developing mossy fiber terminal fields in the rat cerebellar cortex may segregate because of Purkinje cell compartmentation and not competition. J. Comp. Neurol. 359, 197–212 (1995).
Altman, J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J. Comp. Neurol. 145, 399–463 (1972).
Leclerc, N. et al. Synaptophysin expression during synaptogenesis in the rat cerebellar cortex. J. Comp. Neurol. 280, 197–212 (1989).
Arsénio Nunes, M. L., Sotelo C. & Wehrlé, R. Organization of spinocerebellar projection map in three types of agranular cerebellum: Purkinje cells vs. granule cells as organizer element. J. Comp. Neurol. 273, 120–136 (1988).
Eisenman, L. M. & Arlinghaus, L. E. Spinocerebellar projection in the meander tail mutant mouse: organization in the granular posterior lobe and the agranular anterior lobe. Brain Res. 558, 149–152 (1991).
Vig, J., Goldowitz, D., Steindler D. A. & Eisenman L. M. Compartmentation of the reeler cerebellum: segregation and overlap of spinocerebellar and secondary vestibulocerebellar fibers and their target cells. Neuroscience 130, 735–744 (2005).
Goffinet, A. M., So, K. F., Yamamoto, M., Edwards, M. & Caviness, V. S. Jr Architectonic and hodological organization of the cerebellum in reeler mutant mice. Brain Res. 318, 263–276 (1984).
Blatt, G. J. & Eisenman, L. M. Topographic and zonal organization of the olivocerebellar projection in the reeler mutant mouse. J. Comp. Neurol. 267, 603–615 (1988).
Baader, S. L., Vogel, M. W., Sanlioglu, S., Zhang, X. & Oberdick, J. Selective disruption of “late onset” sagittal banding patterns by ectopic expression of engrailed-2 in cerebellar Purkinje cells. J. Neurosci. 19, 5370–5379 (1999).
Tolbert, D. L., Pittman, T., Alisky, J. M. & Clark, B. R. Chronic NMDA receptor blockade or muscimol inhibition of cerebellar cortical neuronal activity alters the development of spinocerebellar afferent topography. Dev. Brain Res. 80, 268–274 (1994).
Arsénio Nunes, M. L. & Sotelo, C. Development of the spinocerebellar system in the postnatal rat. J. Comp. Neurol. 237, 291–306 (1985).
Brodal A. & Kawamura K. Olivocerebellar projection: a review. Adv. Anat. Embryol. Cell Biol. 64, 1–140 (1980).
Buisseret-Delmas, C. & Angaut, P. The cerebellar olivo-corticonuclear connections in the rat. Prog. Neurobiol. 40, 63–87 (1993).
Voogd, J. & Ruigrok, T. J. The organization of the corticonuclear and olivocerebellar climbing fiber projections to the rat cerebellar vermis: the congruence of projection zones and the zebrin pattern. J. Neurocytol. 233, 5–21 (2004).
Groenewegen, H. J. & Voogd, J. The parasagittal zonation within the olivocerebellar projection. I. Climbing fiber distribution in the vermis of the cat cerebellum. J. Comp. Neurol. 174, 417–488 (1977). One of the earlier anatomical studies by Voogd's group that provides important evidence to support the concept of longitudinal zones. The topography of olivocerebellar projections was systematically mapped in cats using degeneration and tritiated leucine methods. Small parts of the inferior olive were found to provide climbing fibre terminations in narrow longitudinally oriented zones in the cerebellar cortex.
Oscarsson, O. Functional units of the cerebellum- sagittal zones and microzones. Trends Neurosci. 2, 144–145 (1979). A short review on the foundational electrophysiological studies undertaken by Oscarsson's group in mapping longitudinal zones and microzones in the cerebellar cortex. It presents the influential hypothesis that cortical microzones and their efferent relay neurons in the cerebellar nuclei might form the basic operational units of the cerebellum.
Trott, J. R. & Armstrong, D. M. The cerebellar corticonuclear projection from lobule Vb/c of the cat anterior lobe: a combined electrophysiological and autoradiographic study. I. Projections from the intermediate region. Exp. Brain Res. 66, 318–338 (1987).
Voogd, J. Olivocerebellar projection in the cat: in the cerebellum — newvistas. Exp. Brain Res. Suppl. 6, 134–161 (1982).
Ekerot, C. F. & Larson, B. Branching of olivary axons to innervate pairs of sagittal zones in the cerebellar anterior lobe of the cat. Exp. Brain Res. 48, 185–198 (1982).
Ruigrok, T. J. Cerebellar nuclei: the olivary connection. Prog. Brain Res. 114, 167–192 (1997).
Pijpers, A., Winkelman, B. H., Bronsing, R. & Ruigrok, T. J. Selective impairment of the cerebellar C1 module involved in rat hind limb control reduces step-dependent modulation of cutaneous reflexes. J. Neurosci. 28, 2179–2189 (2008).
Schonewille, M. et al. Zonal organization of the mouse flocculus: physiology, input, and output. J. Comp. Neurol. 497, 670–682 (2006).
Garwicz, M., Ekerot, C. F. & Jörntell, H. Organizational principles of cerebellar neuronal circuitry. News Physiol. Sci. 13, 26–32 (1998).
Hesslow, G. Correspondence between climbing fibre input and motor output in eyeblink-related areas in cat cerebellar cortex. J. Physiol. 476, 229–244 (1994). An electrophysiological mapping study in decerebrate cats that demonstrates the existence of four spatially separate 'eyeblink' microzones on each side of the cerebellar cortex. It shows a close topographical correspondence between climbing fibre input from the ipsilateral periorbital area and motor output to control movement of the same eye, consistent with cerebellar microzones having functional significance in motor control.
Herrero, L., Pardoe, J. & Apps, R. Pontine and lateral reticular projections to the c1 zone in lobulus simplex and paramedian lobule of the rat cerebellar cortex. Cerebellum 1, 185–199 (2002).
Apps, R. Rostrocaudal branching within the climbing fibre projection to forelimb-receiving areas of the cerebellar cortical C1 zone. J. Comp. Neurol. 419, 193–204 (2000).
Apps, R., Garwicz, M. Precise matching of olivo-cortical divergence and cortico-nuclear convergence between somatotopically corresponding areas in the medial C1 and medial C3 zones of the paravermal cerebellum. Eur. J. Neurosci. 12, 205–214 (2000).
Sugihara, I. & Shinoda, Y. Molecular, topographic, and functional organization of the cerebellar cortex: a study with combined aldolase C and olivocerebellar labeling. J. Neurosci. 24, 8771–8785 (2004).
Gravel, C., Eisenman, L. E., Sasseville, R. & Hawkes, R. Parasagittal organization of the rat cerebellar cortex: a direct correlation between antigenic Purkinje cell bands revealed by mabQ113 and the organization of the olivocerebellar projection. J. Comp. Neurol. 263, 294–310 (1987).
Ji, Z. & Hawkes, R. Topography of Purkinje cell compartments and mossy fiber terminal fields in lobules II and III of the rat cerebellar cortex: spinocerebellar and cuneocerebellar projections. Neuroscience 61, 935–954 (1994).
Voogd, J., Pardoe, J., Ruigrok, T. J. & Apps, R. The distribution of climbing and mossy fiber collateral branches from the copula pyramidis and the paramedian lobule: congruence of climbing fiber cortical zones and the pattern of zebrin banding within the rat cerebellum. J. Neurosci. 23, 4645–4656 (2003). A combined electrophysiological and neuroanatomical tract tracing study in rats, presenting evidence that collateral branches of climbing fibres and mossy fibres associated with a particular longitudinal zone have a congruent spatial organization within the cerebellar cortex that relates to zebrin II stripes, suggesting a common organizational scheme that is consistent with the one-map hypothesis.
Sugihara, I. & Quy, PN. Identification of aldolase C compartments in the mouse cerebellar cortex by olivocerebellar labeling. J. Comp. Neurol. 500, 1076–1092 (2007).
Yaginuma, H., Matsushita, M. Spinocerebellar projections from the thoracic cord in the cat, as studied by anterograde transport of wheat germ agglutinin-horseradish peroxidase. J. Comp. Neurol. 258, 1–27 (1987).
Serapide, M. F., Pantó, M. R., Parenti, R., Zappalá, A. & Cicirata, F. Multiple zonal projections of the basilar pontine nuclei to the cerebellar cortex of the rat. J. Comp. Neurol. 430(4), 471–484 (2001).
Gerrits, N. M., Voogd, J. & Nas, W. S. Cerebellar and olivary projections of the external and rostral internal cuneate nuclei in the cat. Exp. Brain Res. 57, 239–255 (1985).
Wu, H. S., Sugihara, I. & Shinoda, Y. Projection patterns of single mossy fibers originating from the lateral reticular nucleus in the rat cerebellar cortex and nuclei. J. Comp. Neurol. 411(1), 97–118 (1999).
Voogd, J. in Neurobiology of Cerebellar Evolution and Development (ed. Llinás, R.) 493–541 (American Medical Association, Chicago, 1969).
Apps, R., Trott, J. R. & Dietrichs, E. A study of branching from the inferior olive to the x and lateral c1 zones of the cat cerebellum using a combined electrophysiological and retrograde fluorescent double-labelling technique. Exp. Brain Res. 87, 141–152 (1991).
Odeh, F., Ackerley, R., Bjaalie, J. G. & Apps, R. Pontine maps linking somatosensory and cerebellar cortices are in register with climbing fiber somatotopy. J. Neurosci. 25, 5680–5690 (2005).
Pijpers, A. & Ruigrok, T. J. H. Organization of pontocerebellar projections to identified climbing fiber zones in the rat. J. Comp. Neurol. 496, 513–528 (2006).
Shambes, G. M., Gibson, J. M. & Welker, W. Fractured somatotopy in granule cell tactile areas of rat cerebellar hemispheres revealed by micromapping. Brain Behav. Evol. 15, 94–140 (1978). One of the first electrophysiological studies undertaken by Welker's group to systematically map the spatial patterns of tactile projections to the cerebellar cortical granular layer in anaesthetized rats. It presents the important concept of a fractured somatotopical map, in which the cortex comprises a patchy mosaic of receptive fields arising from different body parts.
Chockkan, V. & Hawkes, R. Functional and antigenic maps in the rat cerebellum: Zebrin compartmentation and vibrissal receptive fields in lobule IXa. J. Comp. Neurol. 345, 33–45 (1994).
Hallem, J. S. et al. Spatial correspondence between tactile projection patterns and the distribution of the antigenic Purkinje cell markers anti-zebrin I and anti-zebrin II in the cerebellar folium crus IIa of the rat. Neuroscience 93, 1083–1094 (1999). This study combined immunocytochemistry with electrophysiology to reveal a strong correspondence between the distribution of zebrin II expression and the tactile receptive field map.
Ebner, T. J., Chen, G., Gao, W. & Reinert, K. Optical imaging of cerebellar functional architectures: parallel fiber beams, parasagittal bands and spreading acidification. Prog. Brain Res. 148, 125–138 (2005).
Chen, G., Hanson, C. L. & Ebner, T. J. . Functional parasagittal compartments in the rat cerebellar cortex: an in vivo optical imaging study using neutral red. J. Neurophysiol. 76, 4169–4174 (1996).
Voogd, J. Hess, D. T. & Marani, E. in New Concepts in Cerebellar Neurobiology (ed. King, J. S.) 183–220 (Alan, R. Liss Inc., New York, 1987).
Boegman, R., Parent, A. & Hawkes, R. Zonation in the rat cerebellar cortex: patches of high acetylcholinesterase activity in the granular layer are congruent with Purkinje cell compartments. Brain Res. 448, 237–251 (1988).
Hawkes, R. & Turner, R. W. Compartmentation of NADPH-diaphorase activity in the mouse cerebellar cortex. J. Comp. Neurol. 346, 499–516 (1994).
Schilling, K., Schmidt, H. H. & Baader, S. L. Nitric oxide synthase expression reveals compartments of cerebellar granule cells and suggests a role for mossy fibers in their development. Neuroscience 59, 893–903 (1994).
Leclerc, N., Doré, L., Parent, A. & Hawkes, R. The compartmentalization of the monkey and rat cerebellar cortex: zebrin I and cytochrome oxidase. Brain Res. 506, 70–78 (1990).
Leclerc, N. et al. Synaptophysin expression during synaptogenesis in the rat cerebellar cortex. J. Comp. Neurol. 280, 197–212 (1989).
Marani, E. & Tetteroo, P. A. T. A longitudinal band pattern for the monoclonal human granulocyte antibody B4,3 in the cerebellar external granular layer of the immature rabbit. Histochem. 78, 157–161 (1983).
Karavanova, I., Vasudevan, K., Cheng, J. & Buonanno, A. Novel regional and developmental NMDA receptor expression patterns uncovered in NR2C subunit-beta-galactosidase knock-in mice. Mol. Cell Neurosci. 34, 468–480 (2007).
Sillitoe, R. V., Benson, M. A., Blake, D. J. & Hawkes, R. Abnormal dysbindin expression in cerebellar mossy fiber synapses in the mdx mouse model of Duchenne muscular dystrophy. J. Neurosci. 23, 6576–6585 (2003).
Hawkes, R. An anatomical model of cerebellar modules. Prog. Brain Res. 114, 39–52 (1997).
Hawkes, R., Gallagher R. E. & Ozol K. Blebs in the cerebellar granular layer as a sign of structural inhomogeneity. I. Anterior lobe vermis. Acta Anatomica 158, 205–214 (1997).
Hawkes, R., Gallagher, E. & Ozol, K. Blebs in the cerebellar granular layer as a sign of structural inhomogeneity. II. Posterior lobe vermis. Acta Anatomica 163, 47–55 (1999).
Brown, I. E. & Bower, J. M. Congruence of mossy fiber and climbing fiber tactile projections in the lateral hemispheres of the rat cerebellum. J. Comp. Neurol. 429, 59–70 (2001).
Pijpers, A., Apps, R., Pardoe, J., Voogd, J. & Ruigrok, T. J. Precise spatial relationships between mossy fibers and climbing fibers in rat cerebellar cortical zones. J. Neurosci. 26, 12067–12080 (2006).
Kitai, S. T., Taborikova, H., Tsukahara, N. & Eccles, J. C. The distribution to the cerebellar anterior lobe of the climbing and mossy fiber inputs from the plantar and palmar cutaneous afferents. Exp. Brain Res. 7, 1–10 (1969).
Garwicz, M., Jörntell, H. & Ekerot, C. F. . Cutaneous receptive fields and topography of mossy fibres and climbing fibres projecting to cat cerebellar C3 zone. J. Physiol. 512, 277–293 (1998).
Odeh, F., Ackerley, R., Bjaalie, J. G. & Apps, R. Pontine maps linking somatosensory and cerebellar cortices are in register with climbing fiber somatotopy. J. Neurosci. 25, 5680–5690 (2005).
Nelson, M. E. & Bower, J. M. Brain maps and parallel computers. Trends Neurosci. 13, 403–408 (1990).
Hawkes, R. & Gravel, C. The modular cerebellum. Prog. Neurobiol. 36, 309–327 (1991).
Niven, J. E. & Laughlin, S. B. Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 211, 1792–1804 (2008).
Ito M. The molecular organization of cerebellar long-term depression. Nature Rev. Neurosci. 3, 896–902 (2002).
Barmack, N. H., Qian, Z. & Yoshimura, J. Regional and cellular distribution of protein kinase C in rat cerebellar Purkinje cells. J. Comp. Neurol. 427, 235–254 (2000).
Wadiche, J. I. & Jahr, C. E. Patterned expression of Purkinje cell glutamate transporters controls synaptic plasticity. Nature Neurosci. 8, 1329–1334 (2005).
Tanaka O & Kondo H. Localization of mRNAs for three novel members (beta 3, beta 4 and gamma 2) of phospholipase C family in mature rat brain. Neurosci. Lett. 182, 17–20 (1994).
Sugihara, I. & Shinoda, Y. Molecular, topographic, and functional organization of the cerebellar nuclei: analysis by 3-dimensional mapping of the olivonuclear projection and aldolase C labeling. J. Neurosci. 27, 9696–9710 (2007).
Huang, R. H. & Huang, C. M. An experimental method for measuring the mean length of cerebellar parallel fibers: validation and derivation of a correction factor by computational simulation and probability analysis. Brain Res. Bull. 64, 15–24 (2004).
Braintenberg V. In defense of the cerebellum. Ann. NY Acad. Sci. 978, 175–183 (2002).
Garwicz, G. & Andersson, A. Spread of synaptic activity along parallel fibres in cat cerebellar anterior lobe Exp. Brain Res. 88, 615–622 (1992).
Llinas, R. in The Cerebellum: New Vistas (eds Palay, S. L. & Chan-Palay, V.) 189–194 (Springer, New York, 1982).
Bower, J. M. & Woolston, D. C. Congruence of the spatial organization of tactile projections to the granule cell and Purkinje cell layers of the cerebellar hemispheres of the albino rat: the vertical organization of the cerebellar cortex. J. Neurophysiol. 49, 745–766 (1983).
Cohen, D. & Yarom, Y. Patches of synchronized activity in the cerbellar cortex evoked by mossy fiber stimulation: questioning the role of parallel fibers Proc. Natl. Acad. Sci. 95, 15032–15036 (1998).
Andersson, G. & Nyquist, J. Origin and sagittal termination areas of cerebro-cerebellar climbing fibre paths in the cat. J. Physiol. 337, 257–285 (1983).
Baker, M. R., Javid, M. & Edgley, S. A. Activation of cerebellar climbing fibres to rat cerebellar posterior lobe from motor cortical output pathways. J. Physiol. 536, 825–839 (2001).
Ackerley, R., Pardoe, J. & Apps, R. A novel site of synaptic relay for climbing fibre pathways relaying signals from the motor cortex to the cerebellar cortical C1 zone. J. Physiol. 576, 503–518 (2006).
Arndt, K., Nakagawa, S., Takeichi, M. & Redies, C. Cadherin-defined segments and parasagittal cell ribbons in the developing chicken cerebellum. Mol. Cell Neurosci. 10, 211–228 (1998).
Larouche, M. & Hawkes, R. From clusters to stripes: the developmental origins of adult cerebellar compartmentation. Cerebellum 5, 77–88 (2006).
Armstrong, C. L., Krueger-Naug, A. M. R., Currie, R. W. & Hawkes, R. Expression of heat-shock protein Hsp25 in mouse Purkinje cells during development reveals novel features of cerebellar compartmentation. J. Comp. Neurol. 429, 7–21 (2001).
Apps, R. Columnar organisation of the inferior olive projection to the posterior lobe of the rat cerebellum. J. Comp. Neurol. 302(2), 236–254 (1990).
Jörntell, H., Ekerot, C., Garwicz, M., Luo, X. L. Functional organization of climbing fibre projection to the cerebellar anterior lobe of the rat. J. Physiol. 522(2), 297–309 (2000).
Acknowledgements
The authors thank J. Voogd and M. Usowicz for their comments on a draft manuscript and I. Sugihara for his comments on Table 2. The support of the BBSRC (R.A.), the Canadian Institutes of Health Research (R.H.) and the Wellcome Trust (R.A.) are gratefully acknowledged. Although space restrictions have meant it has only been possible to cite a small fraction of the relevant literature all mistakes and omissions are entirely Richard's fault.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
The conservation of cerebellar zonal architecture (PDF 242 kb)
Related links
Glossary
- Inferior olivary complex
-
Collection of subnuclei located in the ventral medulla oblongata which are the sole source of climbing fibre afferents to the cerebellum.
- Map
-
A systematic spatial representation of anatomical pathways, physiological activity and/or molecular features projected onto the cerebellar cortical surface.
- Aldolase C
-
A brain-specific glycolytic isoenzyme that converts D-fructose 1,6-bisphosphate into glycerone phosphate and D-glyceraldehyde 3-phosphate.
Rights and permissions
About this article
Cite this article
Apps, R., Hawkes, R. Cerebellar cortical organization: a one-map hypothesis. Nat Rev Neurosci 10, 670–681 (2009). https://doi.org/10.1038/nrn2698
Issue Date:
DOI: https://doi.org/10.1038/nrn2698
This article is cited by
-
Cerebellar state estimation enables resilient coupling across behavioural domains
Scientific Reports (2024)
-
Emergence of syntax and word prediction in an artificial neural circuit of the cerebellum
Nature Communications (2024)
-
C1ql1-Bai3 signaling is necessary for climbing fiber synapse formation in mature Purkinje cells in coordination with neuronal activity
Molecular Brain (2023)
-
Hypothermia increases cold-inducible protein expression and improves cerebellar-dependent learning after hypoxia ischemia in the neonatal rat
Pediatric Research (2023)
-
Cerebellar contributions to a brainwide network for flexible behavior in mice
Communications Biology (2023)