Key Points
-
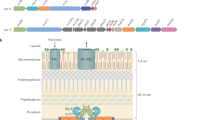
Three families of polytopic proteases with membrane-embedded active sites have been discovered: site 2 proteases (S2Ps) are metalloproteases, presenilin-type aspartyl proteases are represented widely by signal peptide peptidases (SPPs) and rhomboid proteins are serine proteases. Intramembrane proteases have received only limited study in microorganisms until recently.
-
Bacteria generally use S2Ps to regulate membrane-tethered transcription factors, and S2Ps are currently known to modulate envelope lipid composition of Mycobacterium tuberculosis, cholera toxin expression, exopolysaccharide synthesis and pheromone-mediated conjugation.
-
The only known role for a bacterial rhomboid is in quorum sensing by Providencia; rhomboid cleaves to activate TatA of the twin-arginine transporter, although most bacteria have the shorter, rhomboid-independent form of TatA. Bacteria lack SPPs.
-
Protozoan pathogens, including the malaria parasite, use rhomboid proteases to cleave adhesins to dismantle the host–parasite junctions at the end of invasion. Other roles in parasite growth are also beginning to emerge, as are roles in phagocytosis and immune evasion by parasitic amoeba.
-
The only known function of any intramembrane protease in a fungal pathogen centres on S2P, which regulates the sterol regulatory element-binding protein (SREBP) pathway to acclimate the pathogen to hypoxia that is encountered during colonization of host tissues.
-
Viruses do not encode intramembrane proteases, but hepatitis C virus uses a cellular SPP enzyme to cleave core protein, which is important for viral assembly. SPP may also participate in the propagation of other viruses.
-
Further work is required to reveal a complete picture of the roles of different intramembrane proteases in pathogens and to evaluate the therapeutic potential of targeting these enzymes.
Abstract
Proteolysis in cellular membranes to liberate effector domains from their transmembrane anchors is a well-studied regulatory mechanism in animal biology and disease. By contrast, the function of intramembrane proteases in unicellular organisms has received little attention. Recent progress has now established that intramembrane proteases execute pivotal roles in a range of pathogens, from regulating Mycobacterium tuberculosis envelope composition, cholera toxin production, bacterial adherence and conjugation, to malaria parasite invasion, fungal virulence, immune evasion by parasitic amoebae and hepatitis C virus assembly. These advances raise the exciting possibility that intramembrane proteases may serve as targets for combating a wide range of infectious diseases. This Review focuses on summarizing the advances, evaluating the limitations and highlighting the promise of this newly emerging field.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Brown, M. S., Ye, J., Rawson, R. B. & Goldstein, J. L. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell 100, 391–398 (2000).
Wolfe, M. S. & Kopan, R. Intramembrane proteolysis: theme and variations. Science 305, 1119–1123 (2004).
Urban, S. Rhomboid proteins: conserved membrane proteases with divergent biological functions. Genes Dev. 20, 3054–3068 (2006).
Koonin, E. V. et al. The rhomboids: a nearly ubiquitous family of intramembrane serine proteases that probably evolved by multiple ancient horizontal gene transfers. Genome Biol. 4, R19 (2003).
Lemberg, M. K. & Freeman, M. Functional and evolutionary implications of enhanced genomic analysis of rhomboid intramembrane proteases. Genome Res. 17, 1634–1646 (2007).
Kinch, L. N., Ginalski, K. & Grishin, N. V. Site-2 protease regulated intramembrane proteolysis: sequence homologs suggest an ancient signaling cascade. Protein Sci. 15, 84–93 (2006).
Rudner, D. Z., Fawcett, P. & Losick, R. A family of membrane-embedded metalloproteases involved in regulated proteolysis of membrane-associated transcription factors. Proc. Natl Acad. Sci. USA 96, 14765–14770 (1999).
Rather, P. N., Ding, X., Baca-DeLancey, R. R. & Siddiqui, S. Providencia stuartii genes activated by cell-to-cell signaling and identification of a gene required for production or activity of an extracellular factor. J. Bacteriol. 181, 7185–7191 (1999).
An, F. Y., Sulavik, M. C. & Clewell, D. B. Identification and characterization of a determinant (eep) on the Enterococcus faecalis chromosome that is involved in production of the peptide sex pheromone cAD1. J. Bacteriol. 181, 5915–5921 (1999). A library overexpression screen identified an S2P homologue as the protease required for producing several different pheromones in E. faecalis conjugation.
Rawson, R. B. et al. Complementation cloning of S2P, a gene encoding a putative metalloprotease required for intramembrane cleavage of SREBPs. Mol. Cell 1, 47–57 (1997).
Ye, J. et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 6, 1355–1364 (2000).
Kanehara, K., Ito, K. & Akiyama, Y. YaeL (EcfE) activates the sigma(E) pathway of stress response through a site-2 cleavage of anti-sigma(E), RseA. Genes Dev. 16, 2147–2155 (2002).
Alba, B. M., Leeds, J. A., Onufryk, C., Lu, C. Z. & Gross, C. A. DegS and YaeL participate sequentially in the cleavage of RseA to activate the sigma(E)-dependent extracytoplasmic stress response. Genes Dev. 16, 2156–2168 (2002).
Weihofen, A., Binns, K., Lemberg, M. K., Ashman, K. & Martoglio, B. Identification of signal peptide peptidase, a presenilin-type aspartic protease. Science 296, 2215–2218 (2002).
Fluhrer, R. & Haass, C. Signal peptide peptidases and gamma-secretase: cousins of the same protease family? Neurodegener. Dis. 4, 112–116 (2007).
Weihofen, A. & Martoglio, B. Intramembrane-cleaving proteases: controlled liberation of proteins and bioactive peptides. Trends Cell Biol. 13, 71–78 (2003).
Sato, T. et al. Signal peptide peptidase: biochemical properties and modulation by nonsteroidal antiinflammatory drugs. Biochemistry 45, 8649–8656 (2006).
Narayanan, S., Sato, T. & Wolfe, M. S. A C-terminal region of signal peptide peptidase defines a functional domain for intramembrane aspartic protease catalysis. J. Biol. Chem. 282, 20172–20179 (2007).
Ponting, C. P. et al. Identification of a novel family of presenilin homologues. Hum. Mol. Genet. 11, 1037–1044 (2002).
Urban, S., Lee, J. R. & Freeman, M. Drosophila rhomboid-1 defines a family of putative intramembrane serine proteases. Cell 107, 173–182 (2001).
Wang, Y., Zhang, Y. & Ha, Y. Crystal structure of a rhomboid family intramembrane protease. Nature 444, 179–180 (2006).
Wu, Z. et al. Structural analysis of a rhomboid family intramembrane protease reveals a gating mechanism for substrate entry. Nature Struct. Mol. Biol. 13, 1084–1091 (2006).
Ben-Shem, A., Fass, D. & Bibi, E. Structural basis for intramembrane proteolysis by rhomboid serine proteases. Proc. Natl Acad. Sci. USA 104, 462–466 (2007).
Lemieux, M. J., Fischer, S. J., Cherney, M. M., Bateman, K. S. & James, M. N. The crystal structure of the rhomboid peptidase from Haemophilus influenzae provides insight into intramembrane proteolysis. Proc. Natl Acad. Sci. USA 104, 750–754 (2007).
Urban, S. & Freeman, M. Intramembrane proteolysis controls diverse signalling pathways throughout evolution. Curr. Opin. Genet. Dev. 12, 512–518 (2002).
Akiyama, Y., Kanehara, K. & Ito, K. RseP (YaeL), an Escherichia coli RIP protease, cleaves transmembrane sequences. EMBO J. 23, 4434–4442 (2004).
Urban, S. & Wolfe, M. S. Reconstitution of intramembrane proteolysis in vitro reveals that pure rhomboid is sufficient for catalysis and specificity. Proc. Natl Acad. Sci. USA 102, 1883–1888 (2005).
Maegawa, S., Ito, K. & Akiyama, Y. Proteolytic action of GlpG, a rhomboid protease in the Escherichia coli cytoplasmic membrane. Biochemistry 44, 13543–13552 (2005).
Feng, L. et al. Structure of a site-2 protease family intramembrane metalloprotease. Science 318, 1608–1612 (2007).
Baker, R. P., Young, K., Feng, L., Shi, Y. & Urban, S. Enzymatic analysis of a rhomboid intramembrane protease implicates transmembrane helix 5 as the lateral substrate gate. Proc. Natl Acad. Sci. USA 104, 8257–8262 (2007).
Wang, Y. & Ha, Y. Open-cap conformation of intramembrane protease GlpG. Proc. Natl Acad. Sci. USA 104, 2098–2102 (2007).
Urban, S. & Baker, R. P. In vivo analysis reveals substrate-gating mutants of a rhomboid intramembrane protease display increased activity in living cells. Biol. Chem. 389, 1107–1115 (2008).
Urban, S. & Shi, Y. Core principles of intramembrane proteolysis: comparison of rhomboid and site-2 family proteases. Curr. Opin. Struct. Biol. 18, 432–441 (2008).
Osenkowski, P. et al. Cryoelectron microscopy structure of purified γ-secretase at 12 Å resolution. J. Mol. Biol. 385, 642–652 (2009).
Lemberg, M. K. & Freeman, M. Cutting proteins within lipid bilayers: rhomboid structure and mechanism. Mol. Cell 28, 930–940 (2007).
Chen, J. C., Viollier, P. H. & Shapiro, L. A membrane metalloprotease participates in the sequential degradation of a Caulobacter polarity determinant. Mol. Microbiol. 55, 1085–1103 (2005).
Mesak, L. R., Mesak, F. M. & Dahl, M. K. Expression of a novel gene, gluP, is essential for normal Bacillus subtilis cell division and contributes to glucose export. BMC Microbiol. 4, 13 (2004).
King-Lyons, N. D., Smith, K. F. & Connell, T. D. Expression of hurP, a gene encoding a prospective site 2 protease, is essential for heme-dependent induction of bhuR in Bordetella bronchiseptica. J. Bacteriol. 189, 6266–6275 (2007).
Schobel, S., Zellmeier, S., Schumann, W. & Wiegert, T. The Bacillus subtilis σW anti-sigma factor RsiW is degraded by intramembrane proteolysis through YluC. Mol. Microbiol. 52, 1091–1105 (2004).
Denham, E. L., Ward, P. N. & Leigh, J. A. Lipoprotein signal peptides are processed by Lsp and Eep of Streptococcus uberis. J. Bacteriol. 190, 4641–4647 (2008).
Bramkamp, M., Weston, L., Daniel, R. A. & Errington, J. Regulated intramembrane proteolysis of FtsL protein and the control of cell division in Bacillus subtilis. Mol. Microbiol. 62, 580–591 (2006).
Hussain, M., Ichihara, S. & Mizushima, S. Mechanism of signal peptide cleavage in the biosynthesis of the major lipoprotein of the Escherichia coli outer membrane. J. Biol. Chem. 257, 5177–5182 (1982).
Kim, A. C., Oliver, D. C. & Paetzel, M. Crystal structure of a bacterial signal peptide peptidase. J. Mol. Biol. 376, 352–366 (2008).
Grigorova, I. L. et al. Fine-tuning of the Escherichia coli σE envelope stress response relies on multiple mechanisms to inhibit signal-independent proteolysis of the transmembrane anti-sigma factor, RseA. Genes Dev. 18, 2686–2697 (2004).
Glickman, M. S. & Jacobs, W. R. Jr. Microbial pathogenesis of Mycobacterium tuberculosis: dawn of a discipline. Cell 104, 477–485 (2001).
Makinoshima, H. & Glickman, M. S. Regulation of Mycobacterium tuberculosis cell envelope composition and virulence by intramembrane proteolysis. Nature 436, 406–409 (2005). Targeted deletion of an S2P and assessment in an animal model of tuberculosis revealed dysregulation of envelope composition and a dramatic decrease in replication and persistence.
Ramsey, D. M. & Wozniak, D. J. Understanding the control of Pseudomonas aeruginosa alginate synthesis and the prospects for management of chronic infections in cystic fibrosis. Mol. Microbiol. 56, 309–322 (2005).
Qiu, D., Eisinger, V. M., Rowen, D. W. & Yu, H. D. Regulated proteolysis controls mucoid conversion in Pseudomonas aeruginosa. Proc. Natl Acad. Sci. USA 104, 8107–8112 (2007). A screen and subsequent genetic analyses revealed that a multicomponent S2P pathway underlies regulation of alginate biosynthesis.
Bragonzi, A. et al. Sequence diversity of the mucABD locus in Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Microbiology 152, 3261–3269 (2006).
Wood, L. F. & Ohman, D. E. Use of cell wall stress to characterize σ22 (AlgT/U) activation by regulated proteolysis and its regulon in Pseudomonas aeruginosa. Mol. Microbiol. 72, 183–201 (2009).
Matson, J. S., Withey, J. H. & DiRita, V. J. Regulatory networks controlling Vibrio cholerae virulence gene expression. Infect. Immun. 75, 5542–5549 (2007).
Matson, J. S. & DiRita, V. J. Degradation of the membrane-localized virulence activator TcpP by the YaeL protease in Vibrio cholerae. Proc. Natl Acad. Sci. USA 102, 16403–16408 (2005). A genetic screen identified an S2P in the degradation of the transcription factor TcpP as a mechanism for downregulating cholera toxin production.
Weigel, L. M. et al. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science 302, 1569–1571 (2003).
Clewell, D. B., Francia, M. V., Flannagan, S. E. & An, F. Y. Enterococcal plasmid transfer: sex pheromones, transfer origins, relaxases, and the Staphylococcus aureus issue. Plasmid 48, 193–201 (2002).
Chandler, J. R. & Dunny, G. M. Enterococcal peptide sex pheromones: synthesis and control of biological activity. Peptides 25, 1377–1388 (2004).
Flannagan, S. E. & Clewell, D. B. Identification and characterization of genes encoding sex pheromone cAM373 activity in Enterococcus faecalis and Staphylococcus aureus. Mol. Microbiol. 44, 803–817 (2002).
An, F. Y. & Clewell, D. B. Identification of the cAD1 sex pheromone precursor in Enterococcus faecalis. J. Bacteriol. 184, 1880–1887 (2002).
Antiporta, M. H. & Dunny, G. M. ccfA, the genetic determinant for the cCF10 peptide pheromone in Enterococcus faecalis OG1RF. J. Bacteriol. 184, 1155–1162 (2002).
Waters, C. M., Wells, C. L. & Dunny, G. M. The aggregation domain of aggregation substance, not the RGD motifs, is critical for efficient internalization by HT-29 enterocytes. Infect. Immun. 71, 5682–5689 (2003).
Hirt, H., Schlievert, P. M. & Dunny, G. M. In vivo induction of virulence and antibiotic resistance transfer in Enterococcus faecalis mediated by the sex pheromone-sensing system of pCF10. Infect. Immun. 70, 716–723 (2002).
Schlievert, P. M. et al. Aggregation and binding substances enhance pathogenicity in rabbit models of Enterococcus faecalis endocarditis. Infect. Immun. 66, 218–223 (1998).
Chandler, J. R., Hirt, H. & Dunny, G. M. A paracrine peptide sex pheromone also acts as an autocrine signal to induce plasmid transfer and virulence factor expression in vivo. Proc. Natl Acad. Sci. USA 102, 15617–15622 (2005). Genetic ablation of a pheromone signal was used to clarify how dysregulation of the pheromone pathway that leads to virulence occurs after exposure to human serum.
Rather, P. N. & Orosz, E. Characterization of aarA, a pleiotrophic negative regulator of the 2′-N-acetyltransferase in Providencia stuartii. J. Bacteriol. 176, 5140–5144 (1994).
Urban, S., Schlieper, D. & Freeman, M. Conservation of intramembrane proteolytic activity and substrate specificity in eukaryotic and prokaryotic rhomboids. Curr. Biol. 12, 1507–1512 (2002).
Gallio, M., Sturgill, G., Rather, P. & Kylsten, P. A conserved mechanism for extracellular signaling in eukaryotes and prokaryotes. Proc. Natl Acad. Sci. USA 99, 12208–12213 (2002).
Rather, P. N., Parojcic, M. M. & Paradise, M. R. An extracellular factor regulating expression of the chromosomal aminoglycoside 2'-N-acetyltransferase of Providencia stuartii. Antimicrob. Agents Chemother. 41, 1749–1754 (1997).
Stevenson, L. G. et al. Rhomboid protease AarA mediates quorum-sensing in Providencia stuartii by activating TatA of the twin-arginine translocase. Proc. Natl Acad. Sci. USA 104, 1003–1008 (2007). Genetic and biochemical identification of TatA as a substrate, and subsequent epistasis analysis, revealed that rhomboid activates TatA during P. stuartii quorum sensing.
Clemmer, K. M., Sturgill, G. M., Veenstra, A. & Rather, P. N. Functional characterization of Escherichia coli GlpG and additional rhomboid proteins using an aarA mutant of Providencia stuartii. J. Bacteriol. 188, 3415–3419 (2006).
Berks, B. C., Palmer, T. & Sargent, F. The Tat protein translocation pathway and its role in microbial physiology. Adv. Microb. Physiol. 47, 187–254 (2003).
Brossier, F., Jewett, T. J., Lovett, J. L. & Sibley, L. D. C-terminal processing of the toxoplasma protein MIC2 is essential for invasion into host cells. J. Biol. Chem. 278, 6229–6234 (2003).
Carruthers, V. B., Sherman, G. D. & Sibley, L. D. The Toxoplasma adhesive protein MIC2 is proteolytically processed at multiple sites by two parasite-derived proteases. J. Biol. Chem. 275, 14346–14353 (2000).
Brossier, F., Jewett, T. J., Sibley, L. D. & Urban, S. A spatially localized rhomboid protease cleaves cell surface adhesins essential for invasion by Toxoplasma. Proc. Natl Acad. Sci. USA 102, 4146–4151 (2005). Biochemical, expression and subcellular localization analysis revealed a role for one Toxoplasma rhomboid protease in cleaving adhesins during host cell invasion.
Dowse, T. J., Pascall, J. C., Brown, K. D. & Soldati, D. Apicomplexan rhomboids have a potential role in microneme protein cleavage during host cell invasion. Int. J. Parasitol. 35, 747–756 (2005).
Opitz, C. et al. Intramembrane cleavage of microneme proteins at the surface of the apicomplexan parasite Toxoplasma gondii. EMBO J. 21, 1577–1585 (2002).
Howell, S. A. et al. Distinct mechanisms govern proteolytic shedding of a key invasion protein in apicomplexan pathogens. Mol. Microbiol. 57, 1342–1356 (2005).
Zhou, X. W., Blackman, M. J., Howell, S. A. & Carruthers, V. B. Proteomic analysis of cleavage events reveals a dynamic two-step mechanism for proteolysis of a key parasite adhesive complex. Mol. Cell Proteomics 3, 565–576 (2004).
Baker, R. P., Wijetilaka, R. & Urban, S. Two Plasmodium rhomboid proteases preferentially cleave different adhesins implicated in all invasive stages of malaria. PLoS Pathog. 2, e113 (2006). Analysis of rhomboid protease activity revealed two Plasmodium rhomboid proteases cleave adhesins implicated in all stages of parasite invasion.
Dowse, T. J. & Soldati, D. Rhomboid-like proteins in Apicomplexa: phylogeny and nomenclature. Trends Parasitol. 21, 254–258 (2005).
Harris, P. K. et al. Molecular identification of a malaria merozoite surface sheddase. PLoS Pathog. 1, 241–251 (2005).
O'Donnell, R. A. et al. Intramembrane proteolysis mediates shedding of a key adhesin during erythrocyte invasion by the malaria parasite. J. Cell Biol. 174, 1023–1033 (2006). P. falciparum ROM4 subcellular localization, cleavage site mapping and transgenic expression of adhesin mutants together implicate ROM4 in merozoite invasion.
Singh, S., Plassmeyer, M., Gaur, D. & Miller, L. H. Mononeme: a new secretory organelle in Plasmodium falciparum merozoites identified by localization of rhomboid-1 protease. Proc. Natl Acad. Sci. USA 104, 20043–20048 (2007).
Stubbs, J. et al. Molecular mechanism for switching of P. falciparum invasion pathways into human erythrocytes. Science 309, 1384–1387 (2005).
Trasarti, E., Pizzi, E., Pozio, E. & Tosini, F. The immunological selection of recombinant peptides from Cryptosporidium parvum reveals 14 proteins expressed at the sporozoite stage, 7 of which are conserved in other apicomplexa. Mol. Biochem. Parasitol. 152, 159–169 (2007).
Li, J., Zhang, X., Liu, Q., Yin, J. & Yang, J. Eimeria tenella: cloning of a novel Eimeria tenella cDNA encoding a protein related to rhomboid family from F2 hybrid strain. Exp. Parasitol. 113, 215–220 (2006).
Li, X., Chen, H., Oh, S. S. & Chishti, A. H. A presenilin-like protease associated with Plasmodium falciparum micronemes is involved in erythrocyte invasion. Mol. Biochem. Parasitol. 158, 22–31 (2008).
Nyborg, A. C., Ladd, T. B., Jansen, K., Kukar, T. & Golde, T. E. Intramembrane proteolytic cleavage by human signal peptide peptidase like 3 and malaria signal peptide peptidase. FASEB J. 20, 1671–1679 (2006).
Li, X. et al. Plasmodium falciparum signal peptide peptidase is a promising drug target against blood stage malaria. Biochem. Biophys. Res. Commun. 13, 454–459 (2009).
Sheiner, L., Dowse, T. J. & Soldati-Favre, D. Identification of trafficking determinants for polytopic rhomboid proteases in Toxoplasma gondii. Traffic 9, 665–677 (2008).
Brossier, F., Starnes, G. L., Beatty, W. L. & Sibley, L. D. Microneme rhomboid protease TgROM1 is required for efficient intracellular growth of Toxoplasma gondii. Eukaryot. Cell 7, 664–674 (2008). The first genetic knockdown of a rhomboid in any eukaryotic pathogen revealed a function for a non-adhesin-processing rhomboid family member in intracellular growth.
Srinivasan, P., Coppens, I. & Jacobs-Lorena, M. Distinct roles of Plasmodium rhomboid 1 in parasite development and malaria pathogenesis. PLoS Pathog. 5, e1000262 (2009). Targeted deletion of P. berghei ROM1 revealed that parasites complete their entire life cycle, but with decreased efficiency at various steps, were less virulent in rodents and provided protective immunity against subsequent infection.
Baxt, L. A., Baker, R. P., Singh, U. & Urban, S. An Entamoeba histolytica rhomboid protease with atypical specificity cleaves a surface lectin involved in phagocytosis and immune evasion. Genes Dev. 22, 1636–1646 (2008). Biochemical and subcellular localization suggest that the sole amoeba rhomboid protease functions in lectin shedding, phagocytosis and immune evasion.
Petri, W. A. Jr, Haque, R. & Mann, B. J. The bittersweet interface of parasite and host: lectin–carbohydrate interactions during human invasion by the parasite Entamoeba histolytica. Annu. Rev. Microbiol. 56, 39–64 (2002).
Espinosa-Cantellano, M. & Martinez-Palomo, A. Entamoeba histolytica: mechanism of surface receptor capping. Exp. Parasitol. 79, 424–435 (1994).
Hughes, A. L., Todd, B. L. & Espenshade, P. J. SREBP pathway responds to sterols and functions as an oxygen sensor in fission yeast. Cell 120, 831–842 (2005).
Chun, C. D., Liu, O. W. & Madhani, H. D. A link between virulence and homeostatic responses to hypoxia during infection by the human fungal pathogen Cryptococcus neoformans. PLoS Pathog. 3, e22 (2007).
Chang, Y. C., Bien, C. M., Lee, H., Espenshade, P. J. & Kwon-Chung, K. J. Sre1p, a regulator of oxygen sensing and sterol homeostasis, is required for virulence in Cryptococcus neoformans. Mol. Microbiol. 64, 614–629 (2007). Together with Reference 95, this study revealed a role for the SREBP pathway, including the S2P homologue, in the C. neoformans hypoxia response and virulence in animal models.
Hoppe, T., Rape, M. & Jentsch, S. Membrane-bound transcription factors: regulated release by RIP or RUP. Curr. Opin. Cell Biol. 13, 344–348 (2001).
Moradpour, D., Penin, F. & Rice, C. M. Replication of hepatitis C virus. Nature Rev. Microbiol. 5, 453–463 (2007).
Hussy, P., Langen, H., Mous, J. & Jacobsen, H. Hepatitis C virus core protein: carboxy-terminal boundaries of two processed species suggest cleavage by a signal peptide peptidase. Virology 224, 93–104 (1996).
McLauchlan, J., Lemberg, M. K., Hope, G. & Martoglio, B. Intramembrane proteolysis promotes trafficking of hepatitis C virus core protein to lipid droplets. EMBO J. 21, 3980–3988 (2002).
Randall, G. et al. Cellular cofactors affecting hepatitis C virus infection and replication. Proc. Natl Acad. Sci. USA 104, 12884–12889 (2007).
Targett-Adams, P., Hope, G., Boulant, S. & McLauchlan, J. Maturation of hepatitis C virus core protein by signal peptide peptidase is required for virus production. J. Biol. Chem. 283, 16850–16859 (2008).
Okamoto, K. et al. Intramembrane processing by signal peptide peptidase regulates the membrane localization of hepatitis C virus core protein and viral propagation. J. Virol. 82, 8349–8361 (2008). References 102 and 103 used a series of different approaches and overcame prior strain inconsistencies to reveal a direct requirement for core processing by SPP in HCV virus assembly and propagation.
Vauloup-Fellous, C. et al. Signal peptide peptidase-catalyzed cleavage of hepatitis C virus core protein is dispensable for virus budding but destabilizes the viral capsid. J. Biol. Chem. 281, 27679–27692 (2006).
Heimann, M., Roman-Sosa, G., Martoglio, B., Thiel, H. J. & Rumenapf, T. Core protein of pestiviruses is processed at the C terminus by signal peptide peptidase. J. Virol. 80, 1915–1921 (2006).
Targett-Adams, P. et al. Signal peptide peptidase cleavage of GB virus B core protein is required for productive infection in vivo. J. Biol. Chem. 281, 29221–29227 (2006).
Loureiro, J. et al. Signal peptide peptidase is required for dislocation from the endoplasmic reticulum. Nature 441, 894–897 (2006).
Sibley, L. D. Intracellular parasite invasion strategies. Science 304, 248–253 (2004).
Opitz, C. & Soldati, D. 'The glideosome': a dynamic complex powering gliding motion and host cell invasion by Toxoplasma gondii. Mol. Microbiol. 45, 597–604 (2002).
Carruthers, V. & Boothroyd, J. C. Pulling together: an integrated model of Toxoplasma cell invasion. Curr. Opin. Microbiol. 10, 83–89 (2007).
Alexander, D. L., Arastu-Kapur, S., Dubremetz, J. F. & Boothroyd, J. C. Plasmodium falciparum AMA1 binds a rhoptry neck protein homologous to TgRON4, a component of the moving junction in Toxoplasma gondii. Eukaryot. Cell 5, 1169–1173 (2006).
McQuibban, G. A., Saurya, S. & Freeman, M. Mitochondrial membrane remodelling regulated by a conserved rhomboid protease. Nature 423, 537–541 (2003).
Acknowledgements
I apologize to those scientists whose work could not be discussed or cited owing to space limitations. I thank R. Baker for expert help with the illustrations. Work in the Urban laboratory is supported by National Institutes of Health grant R01AI066025, a career award from the Burroughs Wellcome Fund and a Packard Foundation Fellowship for Science and Engineering.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Related links
DATABASES
Entrez Genome Project
FURTHER INFORMATION
Glossary
- SREBP
-
A transcription factor that activates the expression of genes required for cholesterol and lipid biosynthesis in animals.
- Extracytoplasmic function
-
Refers to a range of conditions that are commonly detected by the unfolding of outer membrane proteins, which stimulates proteases that liberate E from the membrane to activate the expression of response genes.
- Metalloprotease
-
An enzyme that cleaves peptide bonds using a bound zinc ion to facilitate hydrolysis. The zinc is usually held in place by two conserved histidines and one acidic residue.
- Aspartyl protease
-
A hydrolytic enzyme that uses two aspartate residues to activate water for cleaving peptide bonds.
- Serine protease
-
An enzyme that uses a serine as a nucleophile for cleavage of peptide bonds. The serine is usually activated by a basic residue, and forms a covalent intermediate with the substrate that is released through attack by water.
- Anti-sigma factor
-
A protein that binds and hinders the transcription-activating function of a bacterial sigma factor.
- Exopolysaccharide
-
A linear polymer of modified sugars that is not generally attached to a bacterium and acts as an extracellular matrix.
- Nosocomial
-
An infection acquired in a hospital.
- Conjugation
-
A physical joining of two bacterial cells for the purpose of transferring genetic material.
- Quorum sensing
-
A cell-to-cell signalling mechanism by which bacteria monitor their population size and react to it accordingly.
- Pleiotrophic
-
The state of having multiple, and seemingly unrelated, phenotypes.
- Biolog
-
A commercial, phenotypic testing method conducted using a large number of standardized conditions.
- Moving junction
-
A specialized adhesive point of contact between the parasite and host that appears as an electron-dense structure in electron microscopic analysis, and traverses the surface of the parasite as a tight ring during invasion of the host
- Merozoite
-
The parasitic form specialized for invasion of and replication within erythrocytes.
- Band 3
-
An integral membrane protein responsible for chloride and bicarbonate exchange on erythrocytes.
- Microneme
-
A specialized, apical, tiny, vesicle-like organelle of apicomplexan parasites that houses adhesins and other proteins for secretion during invasion.
- Gal–GalNAc lectin
-
A protein that is specialized for binding sugars, in this case containing galactose and Nacetylgalactosamine.
- Azole drug
-
A class of anti-fungal drug that inhibits the enzyme lanosterol 14a-demethylase, which is required for ergoesterol biosynthesis.
- RUP
-
A form of proteasome degradation that releases intact domains from the membrane into the cytosol.
- Lipid droplet
-
The lipid storage organelle of cells, in which triacylglycerides in the form of a drop are surrounded by a single leaflet of membrane phospholipid.
Rights and permissions
About this article
Cite this article
Urban, S. Making the cut: central roles of intramembrane proteolysis in pathogenic microorganisms. Nat Rev Microbiol 7, 411–423 (2009). https://doi.org/10.1038/nrmicro2130
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2130
This article is cited by
-
Lactiplantibacillus plantarum as a novel platform for production and purification of integral membrane proteins using RseP as the benchmark
Scientific Reports (2023)
-
MBTPS2, a membrane bound protease, underlying several distinct skin and bone disorders
Journal of Translational Medicine (2021)
-
Ten catalytic snapshots of rhomboid intramembrane proteolysis from gate opening to peptide release
Nature Structural & Molecular Biology (2019)
-
Cytosolic extensions directly regulate a rhomboid protease by modulating substrate gating
Nature (2015)
-
Membrane proximal ectodomain cleavage of MUC16 occurs in the acidifyingGolgi/post-Golgi compartments
Scientific Reports (2015)