Key Points
-
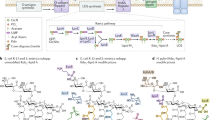
Gram-positive bacteria lack regular outer membranes and instead have thickened peptidoglycan cell walls. Within the fabric of the cell wall, Gram-positive bacteria contain additional cell-wall glycopolymers (CWGs), including at least one membrane-attached (M-CWG) and one peptidoglycan-attached (P-CWG) type. CWG structures are highly diverse and their functions are only partially understood.
-
CWGs differ with regards to their sugar building blocks and non-glycosyl residues. According to net charge, CWGs can be classified into zwitterionic, anionic and uncharged polymers. The classical wall teichoic acids or lipoteichoic acids are zwitterionic because of their negatively charged phosphate and positively charged amino groups (positively charged amino groups are derived in most cases from D-alanine residues). Polyanionic CWGs include, for example, pyruvylated polysaccharides from S-layer-protein-displaying bacilli and their relatives, teichuronic acids and succinylated lipoglycans. Uncharged, often branched CWGs are found, for example, in the cell walls of Mycobacterium tuberculosis and other actinobacteria.
-
Most CWGs seem to have functions in protecting bacterial cell envelopes through the attachment of further protective surface structures, such as S-layer proteins or mycolic acids, or by directly impeding the passage of harmful molecules. Further roles in binding surface proteins and cations, directing the cell-division machinery and enabling biofilm formation by shaping physicochemical surface properties have been described.
-
In addition to bacterial physiology, CWGs can have a range of crucial functions in bacteria–host interactions. These can include: bacterial attachment to host cells, probably through scavenger-receptor-like molecules; induction of proinflammatory responses by Toll-like receptors; activation of complement; and binding of antibodies. Moreover, there is growing evidence that certain zwitterionic glycopolymers can induce major histocompatibility complex class II-dependent activation of T cells, followed by the induction of immunological memory.
-
The pivotal roles of CWGs for Gram-positive viability and/or virulence, together with their easy accessibility, make CWGs promising targets for diagnostics, vaccines and antibiotics. In fact, established diagnostic procedures, such as serological streptococcal differentiation, take advantage of species-specific differences in CWGs. Moreover, promising reports on the use of certain CWGs for active or passive vaccination or of CWG-biosynthetic enzymes as targets for novel antimicrobial compounds indicate the future potential of CWGs for use in new anti-infective strategies.
-
Many topics on CWGs that range from their structural diversity, functions in bacterial physiology and cell division, roles in specific host interactions and usefulness for preventing infections remain major challenges for future research. How CWGs can contribute to bacterial fitness, virulence and host cell tropism, how static or plastic CWG structures are during changing environmental conditions and how CWG biosynthesis is regulated should be investigated more thoroughly using sophisticated glycomics and systems-biology approaches.
Abstract
Abstract | Most Gram-positive bacteria incorporate membrane- or peptidoglycan-attached carbohydrate-based polymers into their cell envelopes. Such cell-wall glycopolymers (CWGs) often have highly variable structures and have crucial roles in protecting, connecting and controlling the major envelope constituents. Further important roles of CWGs in host-cell adhesion, inflammation and immune activation have also been described in recent years. Identifying and harnessing highly conserved or species-specific structural features of CWGs offers excellent opportunities for developing new antibiotics, vaccines and diagnostics for use in the fight against severe infectious diseases, such as sepsis, pneumonia, anthrax and tuberculosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Archibald, A. R., Hancock, I. C. & Harwood, C. R. in Bacillus subtilis and other Gram-positive Bacteria: Physiology and Molecular Genetics (ed. Sonenshein, A. L.) 381–410 (ASM, Washington DC, 1993).
Ghuysen, J. M. & Hakenbeck, R. (eds) Bacterial Cell Wall 1–606 (Elsevier Science, Amsterdam, 1994).
Baddiley, J. Teichoic acids in cell walls and membranes of bacteria. Essays Biochem. 8, 35–77 (1972).
Ward, J. B. Teichoic and teichuronic acids: biosynthesis, assembly and location. Microbiol. Rev. 45, 211–243 (1981).
Sutcliffe, I. C. & Shaw, N. Atypical lipoteichoic acids of Gram-positive bacteria. J. Bacteriol. 173, 7065–7069 (1991).
Fischer, W. in Bacterial Cell wall (eds Ghuysen, J. M. & Hakenbeck, R.) 199–215 (Elsevier Science, Amsterdam, 1994).
Neuhaus, F. C. & Baddiley, J. A continuum of anionic charge: structures and functions of D-alanyl-teichoic acids in Gram-positive bacteria. Microbiol Mol. Biol Rev. 67, 686–723 (2003). A recent comprehensive review on teichoic acids that had a particular emphasis on the role of alanylation.
Brennan, P. J. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis (Edinb.) 83, 91–97 (2003).
Schaffer, C. & Messner, P. The structure of secondary cell wall polymers: how Gram-positive bacteria stick their cell walls together. Microbiology 151, 643–651 (2005).
Fischer, W. in The Handbook of Lipid Research: Glycolipids, Phosphoglycolipids and Sulfoglycolipids (ed. Kates, M.) 123–234 (Plenum, New York,1990).
Naumova, I. B. & Shashkov, A. S. Anionic polymers in cell walls of Gram-positive bacteria. Biochemistry (Mosc.) 62, 809–840 (1997).
Fischer, W. Physiology of lipoteichoic acids in bacteria. Adv. Microb. Physiol. 29, 233–302 (1988). References 10 and 12 by Werner Fischer, the pioneer of LTA research, are the most comprehensive reviews on LTA.
Berg, S., Kaur, D., Jackson, M. & Brennan, P. J. The glycosyltransferases of Mycobacterium tuberculosis — roles in the synthesis of arabinogalactan, lipoarabinomannan, and other glycoconjugates. Glycobiology 17, 35R–56R (2007). A detailed overview of mycobacterial CWGs and CWG-biosynthetic enzymes.
Armstrong, J. J. et al. Composition of teichoic acids from a number of bacterial walls. Nature 184, 247–248 (1959).
Weidenmaier, C. et al. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nature Med. 10, 243–245 (2004). The first demonstration that WTAs are dispensable for the viability of S. aureus , but play an essential part in S. aureus nasal colonization.
D'Elia, M. A. et al. Lesions in teichoic acid biosynthesis in Staphylococcus aureus lead to a lethal gain of function in the otherwise dispensable pathway. J. Bacteriol. 188, 4183–4189 (2006).
Fedtke, I. et al. A Staphylococcus aureus ypfP mutant with strongly reduced lipoteichoic acid (LTA) content: LTA governs bacterial surface properties and autolysin activity. Mol. Microbiol. 65, 1078–1091 (2007).
Bhavsar, A. P. & Brown, E. D. Cell wall assembly in Bacillus subtilis: how spirals and spaces challenge paradigms. Mol. Microbiol. 60, 1077–1090 (2006).
Araki, Y. & Ito, E. Linkage units in cell walls of Gram-positive bacteria. Crit. Rev. Microbiol. 17, 121–135 (1989).
Fischer, W. in New Targets for New Antimicrobial Agents (ed. Hakenbeck, R.) 47–50 (Spektrum Akademischer Verlag, Heidelberg, 1997).
Endl, J., Seidl, H. P., Fiedler, F. & Schleifer, K. H. Chemical composition and structure of the cell wall teichoic acids of staphylococci. Arch. Microbiol. 135, 215–223 (1983).
Potekhina, N. V., Tul'skaya, E. M., Naumova, I. B., Shashkov, A. S. & Evtushenko, L. I. Erythritolteichoic acid in the cell wall of Glycomyces tenuis VKM Ac-1250. Eur. J. Biochem. 218, 371–375 (1993).
Fischer, W., Behr, T., Hartmann, R., Peter-Katalinic, J. & Egge, H. Teichoic acid and lipoteichoic acid of Streptococcus pneumoniae possess identical chain structures. A reinvestigation of teichoid acid (C polysaccharide). Eur. J. Biochem. 215, 851–857 (1993).
Greenberg, J. W., Fischer, W. & Joiner, K. A. Influence of lipoteichoic acid structure on recognition by the macrophage scavenger receptor. Infect. Immun. 64, 3318–3325 (1996).
Shashkov, A. S. et al. Cell wall teichoic acids of streptomycetes of the phenetic cluster 'Streptomyces fulvissimus'. Carbohydr. Res. 341, 796–802 (2006).
Soldo, B., Lazarevic, V., Pagni, M. & Karamata, D. Teichuronic acid operon of Bacillus subtilis 168. Mol. Microbiol. 31, 795–805 (1999).
Choudhury, B. et al. The structure of the major cell wall polysaccharide of Bacillus anthracis is species-specific. J. Biol. Chem. 281, 27932–27941 (2006).
Mesnage, S. et al. Bacterial SLH domain proteins are non-covalently anchored to the cell surface via a conserved mechanism involving wall polysaccharide pyruvylation. EMBO J. 19, 4473–4484 (2000). Demonstration of CWG pyruvylation in B. anthracis and its crucial role in S-layer-protein attachment.
Powell, D. A., Duckworth, M. & Baddiley, J. A membrane-associated lipomannan in micrococci. Biochem. J. 151, 387–397 (1975).
Delmas, C. et al. Comparative structural study of the mannosylated-lipoarabinomannans from Mycobacterium bovis BCG vaccine strains: characterization and localization of succinates. Glycobiology 7, 811–817 (1997).
Freymond, P. P., Lazarevic, V., Soldo, B. & Karamata, D. Poly(glucosyl-N-acetylgalactosamine 1-phosphate), a wall teichoic acid of Bacillus subtilis 168: its biosynthetic pathway and mode of attachment to peptidoglycan. Microbiology 152, 1709–1718 (2006).
Tatituri, R. V. et al. Structural characterization of a partially arabinosylated lipoarabinomannan variant isolated from a Corynebacterium glutamicum ubiA mutant. Microbiology 153, 2621–2629 (2007).
Garton, N. J. et al. A novel lipoarabinomannan from the equine pathogen Rhodococcus equi. Structure and effect on macrophage cytokine production. J. Biol. Chem. 277, 31722–31733 (2002).
Takayama, K., Wang, C. & Besra, G. S. Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculosis. Clin. Microbiol. Rev. 18, 81–101 (2005).
Sutcliffe, I. Lipoarabinomannans — structurally diverse and functionally enigmatic macroamphiphiles of mycobacteria and related actinomycetes. Tuberculosis (Edinb.) 85, 205–206 (2005).
Briken, V., Porcelli, S. A., Besra, G. S. & Kremer, L. Mycobacterial lipoarabinomannan and related lipoglycans: from biogenesis to modulation of the immune response. Mol. Microbiol. 53, 391–403 (2004).
Lazarevic, V. & Karamata, D. The tagGH operon of Bacillus subtilis 168 encodes a two-component ABC transporter involved in the metabolism of two wall teichoic acids. Mol. Microbiol. 16, 345–355 (1995).
Damjanovic, M., Kharat, A. S., Eberhardt, A., Tomasz, A. & Vollmer, W. The essential tacF gene is responsible for the choline-dependent growth phenotype of Streptococcus pneumoniae. J. Bacteriol. 189, 7105–7111 (2007).
Pooley, H. M. & Karamata, D. in Bacterial Cell Wall (eds Ghuysen, J. M. & Hakenbeck, R.) 187–197 (Elsevier Science, Amsterdam, 1994).
Lazarevic, V., Abellan, F. X., Möller, S. B., Karamata, D. & Mauel, C. Comparison of ribitol and glycerol teichoic acid genes in Bacillus subtilis W23 and 168: identical function, similar divergent organization, but different regulation. Microbiology 148, 815–824 (2002).
Qian, Z. et al. Genomic characterization of ribitol teichoic acid synthesis in Staphylococcus aureus: genes, genomic organization and gene duplication. BMC Genomics 7, 74 (2006).
Grundling, A. & Schneewind, O. Synthesis of glycerol phosphate lipoteichoic acid in Staphylococcus aureus. Proc. Natl Acad. Sci. USA 104, 8478–8483 (2007). First description of the long sought LTA polymerase of S. aureus.
Peschel, A. et al. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins and other antimicrobial peptides. J. Biol. Chem. 274, 8405–8410 (1999).
Kristian, S. A. et al. D-alanylation of teichoic acid promotes group A Streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion. J. Bacteriol. 187, 6719–6725 (2005).
D'Elia, M. A., Millar, K. E., Beveridge, T. J. & Brown, E. D. Wall teichoic acid polymers are dispensable for cell viability in Bacillus subtilis. J. Bacteriol. 188, 8313–8316 (2006).
O'Riordan, K. & Lee, J. C. Staphylococcus aureus capsular polysaccharides. Clin. Microbiol. Rev. 17, 218–234 (2004).
Peschel, A. & Sahl, H. G. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nature Rev. Microbiol. 4, 529–536 (2006).
Sieradzki, K. & Tomasz, A. Alterations of cell wall structure and metabolism accompany reduced susceptibility to vancomycin in an isogenic series of clinical isolates of Staphylococcus aureus. J. Bacteriol. 185, 7103–7110 (2003).
Peschel, A., Vuong, C., Otto, M. & Götz, F. The D-alanine residues of Staphylococcus aureus teichoic acids alter the susceptibility to vancomycin and the activity of autolysins. Antimicrob. Agents Chemother. 44, 2845–2847 (2000).
Bera, A. et al. Influence of wall teichoic acid on lysozyme resistance in Staphylococcus aureus. J. Bacteriol. 189, 280–283 (2007). Demonstrates that WTA contributes profoundly to lysozyme resistance.
Gross, M., Cramton, S., Götz, F. & Peschel, A. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect. Immun. 69, 3423–3426 (2001).
Kristian, S. A. et al. Alanylation of teichoic acids protects Staphylococcus aureus against Toll-like receptor 2-dependent host defense in a mouse tissue cage infection model. J. Infect. Dis. 188, 414–423 (2003).
Fabretti, F. et al. Alanine esters of enterococcal lipoteichoic acid play a role in biofilm formation and resistance to antimicrobial peptides. Infect. Immun. 74, 4164–4171 (2006).
Heptinstall, S., Archibald, A. R. & Baddiley, J. Teichoic acids and membrane function in bacteria. Nature 225, 519–521 (1970).
Calamita, H. G., Ehringer, W. D., Koch, A. L. & Doyle, R. J. Evidence that the cell wall of Bacillus subtilis is protonated during respiration. Proc. Natl Acad. Sci. USA 98, 15260–15263 (2001).
Weidenmaier, C., Kristian, S. A. & Peschel, A. Bacterial resistance to antimicrobial host defenses — an emerging target for novel antiinfective strategies? Curr. Drug Targets 4, 643–649 (2003).
Collins, L. V. et al. Staphylococcus aureus strains lacking D-alanine modifications of teichoic acids are highly susceptible to human neutrophil killing and are virulence attenuated in mice. J. Infect. Dis. 186, 214–219 (2002).
Koprivnjak, T., Peschel, A., Gelb, M. H., Liang, N. S. & Weiss, J. P. Role of charge properties of bacterial envelope in bactericidal action of human Group IIA phospholipase A2 against Staphylococcus aureus. J. Biol. Chem. 277, 47636–47644 (2002).
Neuhaus, F. C., Heaton, M. P., Debabov, D. V. & Zhang, Q. The dlt operon in the biosynthesis of D-alanyl-lipoteichoic acid in Lactobacillus casei. Microb. Drug Resist. 2, 77–84 (1996).
Li, M. et al. Gram-positive three-component antimicrobial peptide-sensing system. Proc. Natl Acad. Sci. USA 104, 9469–9474 (2007). First description of regulated teichoic acid modification in response to challenge by antimicrobial peptides.
Herbert, S. et al. Molecular basis of resistance to muramidase and cationic antimicrobial peptide activity of lysozyme in staphylococci. PLoS Pathog. 3, 981–994 (2007).
Li, M. et al. The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol. Microbiol. 66, 1136–1147 (2007).
Jordan, S., Hutchings, M. I. & Mascher, T. Cell envelope stress response in Gram-positive bacteria. FEMS Microbiol. Rev. 32, 107–146 (2008).
Koprivnjak, T. et al. Cation-induced transcriptional regulation of the dlt operon of Staphylococcus aureus. J. Bacteriol. 188, 3622–3630 (2006).
Bergmann, S. & Hammerschmidt, S. Versatility of pneumococcal surface proteins. Microbiology 152, 295–303 (2006).
Jonquieres, R., Bierne, H., Fiedler, F., Gounon, P. & Cossart, P. Interaction between the protein InlB of Listeria monocytogenes and lipoteichoic acid: a novel mechanism of protein association at the surface of Gram-positive bacteria. Mol. Microbiol. 34, 902–914 (1999).
Bierbaum, G. & Sahl, H. G. Autolytic system of Staphylococcus simulans 22: influence of cationic peptides on activity of N-acetylmuramoyl-L-alanine amidase. J. Bacteriol. 169, 5452–5458 (1987). Demonstration of binding and regulation of staphylococcal autolysins by teichoic acids.
Giudicelli, S. & Tomasz, A. Attachment of pneumococcal autolysin to wall teichoic acids, an essential step in enzymatic wall degradation. J. Bacteriol. 158, 1188–1190 (1984).
Park, J. T., Shaw, D. R., Chatterjee, A. N., Mirelman, D. & Wu, T. Mutants of staphylococci with altered cell walls. Ann. NY Acad. Sci. 236, 54–62 (1974).
Wendlinger, G., Loessner, M. J. & Scherer, S. Bacteriophage receptors on Listeria monocytogenes cells are the N-acetylglucosamine and rhamnose substituents of teichoic acids or the peptidoglycan itself. Microbiology 142, 985–992 (1996).
Lopez, R., Garcia, E., Garcia, P., Ronda, C. & Tomasz, A. Choline-containing bacteriophage receptors in Streptococcus pneumoniae. J. Bacteriol. 151, 1581–1590 (1982).
Bentley, S. D. et al. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2, e31 (2006).
Vinogradov, E., Sadovskaya, I., Li, J. & Jabbouri, S. Structural elucidation of the extracellular and cell-wall teichoic acids of Staphylococcus aureus MN8m, a biofilm forming strain. Carbohydr. Res. 341, 738–743 (2006).
Sadovskaya, I., Vinogradov, E., Li, J. & Jabbouri, S. Structural elucidation of the extracellular and cell-wall teichoic acids of Staphylococcus epidermidis RP62A, a reference biofilm-positive strain. Carbohydr. Res. 339, 1467–1473 (2004).
Huebner, J. et al. Isolation and chemical characterization of a capsular polysaccharide antigen shared by clinical isolates of Enterococcus faecalis and vancomycin-resistant Enterococcus faecium. Infect. Immun. 67, 1213–1219 (1999).
Molinari, A. et al. Preservation of capsular material of streptococcal cells by specific lectins determined by immunoelectron microscopy. Histochem. J. 20, 526–530 (1988).
Wittkowski, M. et al. Capsular arabinomannans from Mycobacterium avium with morphotype-specific structural differences but identical biological activity. J. Biol. Chem. 282, 19103–19112 (2007).
Weidenmaier, C. et al. Differential roles of sortase-anchored surface proteins and wall teichoic acid in Staphylococcus aureus nasal colonization. Int. J. Med. Microbiol. 24 Jan 2008 (doi:10.1016/jijmm.2007. 11.006). First evidence for a role of scavenger receptor-like molecules in WTA-mediated binding of S. aureus to epithelial cells.
Weidenmaier, C. et al. Lack of wall teichoic acids in Staphylococcus aureus leads to reduced interactions with endothelial cells and to attenuated virulence in a rabbit model of endocarditis. J. Infect. Dis. 191, 1771–1777 (2005).
Weidenmaier, C. et al. DltABCD- and MprF-mediated cell envelope modifications of Staphylococcus aureus confer resistance to platelet microbicidal proteins and contribute to virulence in a rabbit endocarditis model. Infect. Immun. 73, 8033–8038 (2005).
Abachin, E. et al. Formation of D-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. Mol. Microbiol. 43, 1–14 (2002).
Doran, K. S. et al. Group B Streptococcus blood–brain barrier invasion depends upon proper cell surface anchoring of lipoteichoic acid. J. Clin. Invest. 115, 2499–2507 (2005).
Koppel, E. A. et al. Identification of the mycobacterial carbohydrate structure that binds the C-type lectins DC-SIGN, L-SIGN and SIGNR1. Immunobiology 209, 117–127 (2004).
Peiser, L., Mukhopadhyay, S. & Gordon, S. Scavenger receptors in innate immunity. Curr. Opin. Immunol. 14, 123–128 (2002).
Pluddemann, A., Mukhopadhyay, S. & Gordon, S. The interaction of macrophage receptors with bacterial ligands. Expert. Rev. Mol. Med. 8, 1–25 (2006). A comprehensive overview of scavenger receptors and lectins that bind CWGs or other bacterial glycopolymers.
Goldstein, J. L., Ho, Y. K., Brown, M. S., Innerarity, T. L. & Mahley, R. W. Cholesteryl ester accumulation in macrophages resulting from receptor-mediated uptake and degradation of hypercholesterolemic canine β-very low density lipoproteins. J. Biol. Chem. 255, 1839–1848 (1980).
Murphy, J. E., Tedbury, P. R., Homer-Vanniasinkam, S., Walker, J. H. & Ponnambalam, S. Biochemistry and cell biology of mammalian scavenger receptors. Atherosclerosis 182, 1–15 (2005).
Dunne, D. W., Resnick, D., Greenberg, J., Krieger, M. & Joiner, K. A. The type I macrophage scavenger receptor binds to Gram-positive bacteria and recognizes lipoteichoic acid. Proc. Natl Acad. Sci. USA 91, 1863–1867 (1994).
Thomas, C. A. et al. Protection from lethal Gram-positive infection by macrophage scavenger receptor-dependent phagocytosis. J. Exp. Med. 191, 147–156 (2000).
Elomaa, O. et al. Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell 80, 603–609 (1995).
van der Laan, L. J. et al. Regulation and functional involvement of macrophage scavenger receptor MARCO in clearance of bacteria in vivo. J. Immunol. 162, 939–947 (1999).
Jiang, Y., Oliver, P., Davies, K. E. & Platt, N. Identification and characterization of murine SCARA5, a novel class A scavenger receptor that is expressed by populations of epithelial cells. J. Biol. Chem. 281, 11834–11845 (2006).
Nakamura, K., Funakoshi, H., Miyamoto, K., Tokunaga, F. & Nakamura, T. Molecular cloning and functional characterization of a human scavenger receptor with C-type lectin (SRCL), a novel member of a scavenger receptor family. Biochem. Biophys. Res. Commun. 280, 1028–1035 (2001).
Shimaoka, T. et al. LOX-1 supports adhesion of Gram-positive and Gram-negative bacteria. J. Immunol. 166, 5108–5114 (2001).
Shimaoka, T. et al. Cutting edge: SR-PSOX/CXC chemokine ligand 16 mediates bacterial phagocytosis by APCs through its chemokine domain. J. Immunol. 171, 1647–1651 (2003).
Adachi, H. & Tsujimoto, M. FEEL-1, a novel scavenger receptor with in vitro bacteria-binding and angiogenesis-modulating activities. J. Biol. Chem. 277, 34264–34270 (2002).
Cundell, D. R., Gerard, N. P., Gerard, C., Idanpaan-Heikkila, I. & Tuomanen, E. I. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 377, 435–438 (1995).
Medzhitov, R. Toll-like receptors and innate immunity. Nature Rev. Immunol. 1, 135–145 (2001).
Akira, S. & Takeda, K. Toll-like receptor signalling. Nature Rev. Immunol. 4, 499–511 (2004).
Morath, S., Geyer, A. & Hartung, T. Structure–function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J. Exp. Med. 193, 393–398 (2001).
Han, S. H., Kim, J. H., Martin, M., Michalek, S. M. & Nahm, M. H. Pneumococcal lipoteichoic acid (LTA) is not as potent as staphylococcal LTA in stimulating Toll-like receptor 2. Infect. Immun. 71, 5541–5548 (2003).
Henneke, P. et al. Role of lipoteichoic acid in the phagocyte response to group B Streptococcus. J. Immunol. 174, 6449–6455 (2005).
Hermann, C. et al. Cytokine induction by purified lipoteichoic acids from various bacterial species — role of LBP, sCD14, CD14 and failure to induce IL-12 and subsequent IFN-γ release. Eur. J. Immunol. 32, 541–551 (2002).
Hoebe, K. et al. CD36 is a sensor of diacylglycerides. Nature 433, 523–527 (2005).
Deininger, S. et al. Definition of structural prerequisites for lipoteichoic acid-inducible cytokine induction by synthetic derivatives. J. Immunol. 170, 4134–4138 (2003).
Deininger, S. et al. Definition of the cytokine inducing minimal structure of lipoteichoic acid using synthetic derivatives. Clin. Vaccine Immunol. 14, 1629–1633 (2007). Defined structural requirements for the proinflammatory activity of LTA using synthetic LTA variants.
Hashimoto, M. et al. Not lipoteichoic acid but lipoproteins appear to be the dominant immunobiologically active compounds in Staphylococcus aureus. J. Immunol. 177, 3162–3169 (2006).
Hashimoto, M. et al. Evidence of immunostimulating lipoprotein existing in the natural lipoteichoic acid fraction. Infect. Immun. 75, 1926–1932 (2007).
Sugawara, I. et al. Mycobacterial infection in TLR2 and TLR6 knockout mice. Microbiol. Immunol. 47, 327–336 (2003).
Tapping, R. I. & Tobias, P. S. Mycobacterial lipoarabinomannan mediates physical interactions between TLR1 and TLR2 to induce signaling. J. Endotoxin Res. 9, 264–268 (2003).
Means, T. K. et al. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J. Immunol. 163, 6748–6755 (1999).
Savedra, R. Jr, Delude, R. L., Ingalls, R. R., Fenton, M. J. & Golenbock, D. T. Mycobacterial lipoarabinomannan recognition requires a receptor that shares components of the endotoxin signaling system. J. Immunol. 157, 2549–2554 (1996).
Quesniaux, V. J. et al. Toll-like receptor 2 (TLR2)-dependent-positive and TLR2-independent-negative regulation of proinflammatory cytokines by mycobacterial lipomannans. J. Immunol. 172, 4425–4434 (2004).
Wieland, C. W. et al. Non-mannose-capped lipoarabinomannan induces lung inflammation via toll-like receptor 2. Am. J. Respir. Crit. Care Med. 170, 1367–1374 (2004).
Nigou, J., Zelle-Rieser, C., Gilleron, M., Thurnher, M. & Puzo, G. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J. Immunol. 166, 7477–7485 (2001).
Geijtenbeek, T. B. et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197, 7–17 (2003).
Gringhuis, S. I. et al. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-κβ. Immunity 26, 605–616 (2007).
McGreal, E. P., Martinez-Pomares, L. & Gordon, S. Divergent roles for C-type lectins expressed by cells of the innate immune system. Mol. Immunol. 41, 1109–1121 (2004).
Takahashi, M., Mori, S., Shigeta, S. & Fujita, T. Role of MBL-associated serine protease (MASP) on activation of the lectin complement pathway. Adv. Exp. Med. Biol. 598, 93–104 (2007).
Endo, Y., Matsushita, M. & Fujita, T. Role of ficolin in innate immunity and its molecular basis. Immunobiology 212, 371–379 (2007).
Polotsky, V. Y., Fischer, W., Ezekowitz, A. B. & Joiner, K. A. Interactions of human mannose-binding protein with lipoteichoic acids. Infect. Immun. 64, 380–383 (1996).
Polotsky, V. Y., Belisle, J. T., Mikusova, K., Ezekowitz, R. A. & Joiner, K. A. Interaction of human mannose-binding protein with Mycobacterium avium. J. Infect. Dis. 175, 1159–1168 (1997).
Lynch, N. J. et al. L-ficolin specifically binds to lipoteichoic acid, a cell wall constituent of Gram-positive bacteria, and activates the lectin pathway of complement. J. Immunol. 172, 1198–1202 (2004).
van de Wetering, J. K. et al. Characteristics of surfactant protein A and D binding to lipoteichoic acid and peptidoglycan, 2 major cell wall components of Gram-positive bacteria. J. Infect. Dis. 184, 1143–1151 (2001).
Sidobre, S., Nigou, J., Puzo, G. & Riviere, M. Lipoglycans are putative ligands for the human pulmonary surfactant protein A attachment to mycobacteria. Critical role of the lipids for lectin-carbohydrate recognition. J. Biol. Chem. 275, 2415–2422 (2000).
Ferguson, J. S., Voelker, D. R., McCormack, F. X. & Schlesinger, L. S. Surfactant protein D binds to Mycobacterium tuberculosis bacilli and lipoarabinomannan via carbohydrate-lectin interactions resulting in reduced phagocytosis of the bacteria by macrophages. J. Immunol. 163, 312–321 (1999).
Koppel, E. A., Saeland, E., de Cooker, D. J. M., van, Kooyk, Y. & Geijtenbeek, T. B. H. DC-SIGN specifically recognizes Streptococcus pneumoniae serotypes 3 and 14. Immunobiology 210, 203–210 (2005).
Kumar, A., Ray, P., Kanwar, M., Sharma, M. & Varma, S. A comparative analysis of antibody repertoire against Staphylococcus aureus antigens in patients with deep-seated versus superficial staphylococcal infections. Int. J. Med. Sci. 2, 129–136 (2005).
Verbrugh, H. A., Peters, R., Rozenberg-Arska, M., Peterson, P. K. & Verhoef, J. Antibodies to cell wall peptidoglycan of Staphylococcus aureus in patients with serious staphylococcal infections. J. Infect. Dis. 144, 1–9 (1981).
Verhoef, J., Musher, D. M., Spika, J. S., Verbrugh, H. A. & Jaspers, F. C. The effect of staphylococcal peptidoglycan on polymorphonuclear leukocytes in vitro and in vivo. Scand. J. Infect. Dis. Suppl. 41, 79–86 (1983).
Theilacker, C. et al. Opsonic antibodies to Enterococcus faecalis strain 12030 are directed against lipoteichoic acid. Infect. Immun. 74, 5703–5712 (2006).
Theilacker, C., Krueger, W. A., Kropec, A. & Huebner, J. Rationale for the development of immunotherapy regimens against enterococcal infections. Vaccine 22 (Suppl. 1), 31–38 (2004).
Walsh, S., Kokai-Kun, J., Shah, A. & Mond, J. Extended nasal residence time of lysostaphin and an anti-staphylococcal monoclonal antibody by delivery in semisolid or polymeric carriers. Pharm. Res. 21, 1770–1775 (2004).
Barrett, D. J. Human immune responses to polysaccharide antigens: an analysis of bacterial polysaccharide vaccines in infants. Adv. Pediatr. 32, 139–158 (1985).
Coutinho, A., Moller, G., Anderson, J. & Bullock, W. W. In vitro activation of mouse lymphocytes in serum-free medium: effect of T and B cell mitogens on proliferation and antibody synthesis. Eur. J. Immunol. 3, 299–306 (1973).
Oosterhuis-Kafeja, F., Beutels, P. & Van Damme, P. Immunogenicity, efficacy, safety and effectiveness of pneumococcal conjugate vaccines (1998–2006). Vaccine 25, 2194–2212 (2007).
Lee, J. C. Development of antistaphylococcal vaccines. Curr. Infect. Dis. Rep. 3, 517–524 (2001).
Lee, C. J., Lee, L. H., Lu, C. S. & Wu, A. Bacterial polysaccharides as vaccines — immunity and chemical characterization. Adv. Exp. Med. Biol. 491, 453–471 (2001).
Mazmanian, S. K. & Kasper, D. L. The love–hate relationship between bacterial polysaccharides and the host immune system. Nature Rev. Immunol. 6, 849–858 (2006).
Tzianabos, A. O., Onderdonk, A. B., Rosner, B., Cisneros, R. L. & Kasper, D. L. Structural features of polysaccharides that induce intra-abdominal abscesses. Science 262, 416–419 (1993).
Kalka-Moll, W. M. et al. Zwitterionic polysaccharides stimulate T cells by MHC class II-dependent interactions. J. Immunol. 169, 6149–6153 (2002).
Tzianabos, A. O., Wang, J. Y. & Lee, J. C. Structural rationale for the modulation of abscess formation by Staphylococcus aureus capsular polysaccharides. Proc. Natl Acad. Sci. USA 98, 9365–9370 (2001). First evidence that S. aureus WTA can activate T cells.
McLoughlin, R. M. et al. CD4+ T cells and CXC chemokines modulate the pathogenesis of Staphylococcus aureus wound infections. Proc. Natl Acad. Sci. USA 103, 10408–10413 (2006).
Prigozy, T. I. et al. The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immunity 6, 187–197 (1997).
Brigl, M. & Brenner, M. B. CD1: antigen presentation and T cell function. Annu. Rev. Immunol. 22, 817–890 (2004).
Lancefield, R. C. A serological differentiation of human and other groups of hemolytic streptococci. J. Exp. Med. 57, 571–595 (1933).
Lancefield, R. C. & Freimer, E. H. Type-specific polysaccharide antigens of group B streptococci. J. Hyg. (Lond.) 64, 191–203 (1966).
McCarty, M. & Lancefield, R. C. Variation in the group-specific carbohydrate of group A streptococci. I. Immunochemical studies on the carbohydrates of variant strains. J. Exp. Med. 102, 11–28 (1955).
Pantucek, R. et al. Identification of bacteriophage types and their carriage in Staphylococcus aureus. Arch. Virol. 149, 1689–1703 (2004).
Leoff, C. et al. Cell wall carbohydrate compositions of strains from the B. cereus group of species correlate with phylogenetic relatedness. J. Bacteriol. 190, 112–121 (2008). Demonstrates that the P-CWG polymer of B. anthracis has a species-specific structure, which confirms its potential for diagnostic purposes.
Weisman, L. E. Antibody for the prevention of neonatal noscocomial staphylococcal infection: a review of the literature. Arch. Pediatr. 14 (Suppl 1), 31–34 (2007).
Mikusova, K., Slayden, R. A., Besra, G. S. & Brennan, P. J. Biogenesis of the mycobacterial cell wall and the site of action of ethambutol. Antimicrob. Agents Chemother. 39, 2484–2489 (1995).
Soldo, B., Lazarevic, V. & Karamata, D. tagO is involved in the synthesis of all anionic cell-wall polymers in Bacillus subtilis 168. Microbiology 148, 2079–2087 (2002).
Ginsberg, C., Zhang, Y. H., Yuan, Y. & Walker, S. In vitro reconstitution of two essential steps in wall teichoic acid biosynthesis. ACS Chem. Biol. 1, 25–28 (2006).
Bhavsar, A. P., Truant, R. & Brown, E. D. The TagB protein in Bacillus subtilis 168 is an intracellular peripheral membrane protein that can incorporate glycerol phosphate onto a membrane-bound acceptor in vitro. J. Biol. Chem. 280, 36691–36700 (2005).
Brown, S., Zhang, Y. H. & Walker, S. A revised pathway proposed for Staphylococcus aureus wall teichoic acid biosynthesis based on in vitro reconstitution of the intracellular steps. Chem. Biol. 15, 12–21 (2008).
May, J. J. et al. Inhibition of the D-alanine:D-alanyl carrier protein ligase from Bacillus subtilis increases the bacterium's susceptibility to antibiotics that target the cell wall. FEBS J. 272, 2993–3003 (2005). Describes the first inhibitor of the teichoic acid D -alanylation pathway and its potential for antimicrobial therapy.
Escaich, S. et al. Discovery of new Gram-positive antivirulence drugs: the first antivirulence molecule active in vivo. Abstr. Intersci. Conf. Antimicrob. Agents Chemother. F2–958 (2007).
Fischetti, V. A., Nelson, D. & Schuch, R. Reinventing phage therapy: are the parts greater than the sum? Nature Biotechnol. 24, 1508–1511 (2006).
Nagarajan, V. & Elasri, M. O. SAMMD: Staphylococcus aureus microarray meta-database. BMC Genomics 8, 351 (2007).
Schaffer, C. & Messner, P. Surface-layer glycoproteins: an example for the diversity of bacterial glycosylation with promising impacts on nanobiotechnology. Glycobiology 14, 31R–42R (2004).
Bensing, B. A., Takamatsu, D. & Sullam, P. M. Determinants of the streptococcal surface glycoprotein GspB that facilitate export by the accessory Sec system. Mol. Microbiol. 58, 1468–1481 (2005).
Hitchen, P. G. & Dell, A. Bacterial glycoproteomics. Microbiology 152, 1575–1580 (2006).
Mehta, A. S. et al. Synthesis and antigenic analysis of the BclA glycoprotein oligosaccharide from the Bacillus anthracis exosporium. Chemistry 12, 9136–9149 (2006).
Ratledge, C. & Wilkinson, S. G. Microbial Lipids 1–963 (Academic, London, 1988).
Karakousis, P. C., Bishai, W. R. & Dorman, S. E. Mycobacterium tuberculosis cell envelope lipids and the host immune response. Cell. Microbiol. 6, 105–116 (2004).
Dmitriev, B. A., Toukach, F. V., Holst, O., Rietschel, E. T. & Ehlers, S. Tertiary structure of Staphylococcus aureus cell wall murein. J. Bacteriol. 186, 7141–7148 (2004).
Vollmer, W., Blanot, D. & de Pedro, M. A. Peptidoglycan structure and architecture. FEMS Microbiol Rev. 8 Jan 2008 (doi: 10.1111/j.1574-6976.2007.00094.x).
Paulson, J. C., Blixt, O. & Collins, B. E. Sweet spots in functional glycomics. Nature Chem. Biol. 2, 238–248 (2006).
Comstock, L. E. & Kasper, D. L. Bacterial glycans: key mediators of diverse host immune responses. Cell 126, 847–850 (2006).
Cobb, B. A., Wang, Q., Tzianabos, A. O. & Kasper, D. L. Polysaccharide processing and presentation by the MHCII pathway. Cell 117, 677–687 (2004). Describes how zwitterionic B. fragilis capsular polysaccharides are processed and presented by MHC class II molecules to T cells.
Ruiz-Perez, B. et al. Modulation of surgical fibrosis by microbial zwitterionic polysaccharides. Proc. Natl Acad. Sci. USA 102, 16753–16758 (2005).
Tzianabos, A. O., Kasper, D. L. & Onderdonk, A. B. Structure and function of Bacteroides fragilis capsular polysaccharides: relationship to induction and prevention of abscesses. Clin. Infect. Dis. 20 (Suppl. 2), 132–140 (1995).
Acknowledgements
The authors thank J. Lee, E. Kannenberg and J. Kokai-Kun for helpful discussions and H. Hölscher for critically reading the manuscript. A.P. is supported by grants from the German Research Foundation (TR-SFB34, FOR449, GRK685, SFB766 and SFB685), the European Union (LSHM-CT-2004-512093), the German Ministry of Education and Research (NGFN2 and SKINSTAPH) and the IZKF programme of the Medical Faculty, University of Tübingen. C.W. is supported by grants from the German Research Foundation (WE 4291/1-1 and SPP1130).
Author information
Authors and Affiliations
Related links
Related links
DATABASES
Entrez Genome Project
FURTHER INFORMATION
Glossary
- Capsular polysaccharide
-
A glycopolymer, usually of variable composition and structure, that forms capsule-like protective layers around microbial cells.
- S-layer protein
-
Forms a crystalline two-dimensional lattice on microbial-cell surfaces.
- Mycolic acid
-
Very-long-chain fatty acid that contains up to 60 carbon atoms and unusual modifications that form an outer-membrane-like layer on the surface of mycobacteria and their relatives. Mycolic acids are responsible for mycobacterial surface hydrophobicity and resistance to most conventional antibiotics.
- Teichoic acid
-
A cell-envelope glycopolymer that is composed of many identical sugar-phosphate repeating units, which are usually modified with d-alanine and additional sugars.
- Lipoteichoic acid (LTA)
-
A teichoic acid species that is connected to membrane glycolipids. The stereochemistry of LTAs and the biosynthetic origin of the glycerolphosphates are different from those of wall teichoic acids, which have glycerol-phosphate backbones.
- Teichuronic acid
-
A teichoic acid-like polymer that lacks phosphate groups and possesses polyanionic properties because of the presence of uronic acid-containing repeating units.
- Uronic acid
-
A sugar-derived acid, such as glucuronic acid or galacturonic acid.
- Zwitterionic
-
The occurrence of both negatively and positively charged groups in a molecule.
- Bacteriocin
-
A bacterially produced, small, heat-stable peptide that is active against other bacteria and to which the producer has a specific immunity mechanism. Bacteriocins can have a narrow or broad target spectrum.
- Defensin
-
A cationic peptide that is produced by the innate immune system, and kills bacteria, for example, by disrupting the phospholipid bilayer.
- Pathogen-associated molecular pattern
-
A small molecular motif that is conserved across microbial species and engages innate immune receptors (for example, Toll-like receptors).
- Pattern-recognition receptor
-
A host receptor (such as a Toll-like receptor) that can sense pathogen-associated molecular patterns and initiate signalling cascades that lead to an innate immune response.
- Type I transmembrane protein
-
A protein which contains a single membrane-spanning domain that has its carboxyl terminus orientated towards the cytoplasm and its amino terminus orientated towards the lumen of membrane compartments or in an extracellular direction.
Rights and permissions
About this article
Cite this article
Weidenmaier, C., Peschel, A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat Rev Microbiol 6, 276–287 (2008). https://doi.org/10.1038/nrmicro1861
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro1861
This article is cited by
-
Structural insights into the transporting and catalyzing mechanism of DltB in LTA D-alanylation
Nature Communications (2024)
-
Cross-regulation of Aps-promoters in Lacticaseibacillus paracasei by the PsdR response regulator in response to lantibiotics
Scientific Reports (2024)
-
Bacteria can shed a layer when phages turn up the heat
Nature Microbiology (2023)
-
Assembly of Bacterial Surface Glycopolymers as an Antibiotic Target
Journal of Microbiology (2023)
-
Gut health benefit and application of postbiotics in animal production
Journal of Animal Science and Biotechnology (2022)