Key Points
-
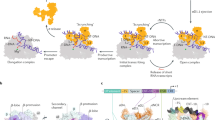
The DNA-dependent multi-subunit RNA polymerase is the central component in the regulation of transcription initiation in bacteria. Sigma factors bind to the RNA polymerase to produce a holoenzyme that is competent for transcription. There are multiple factors that determine the level of transcription of a particular gene in a bacterium.
-
The crucial sequence elements of a bacterial promoter are described, including the −35 element, the −10 element, the extended −10 element and the UP element.
-
The role played by sigma factors in bacterial gene regulation is briefly outlined.
-
Small ligands, like ppGpp, can influence the level of transcription of certain genes.
-
Transcription factors can either activate or repress transcription by several mechanisms, but all of these mechanisms involve either optimizing the interaction of the RNA polymerase holoenzyme with the promoter (activation) or preventing the RNA polymerase holoenzyme from binding to the promoter (repression).
-
The organisation of the bacterial chromosome — although still poorly understood — contributes to the regulation of bacterial transcription initiation.
-
Promoters are frequently regulated by changes in environmental or physiological conditions. It is rare for a promoter to be regulated in response to a single environmental or physiological factor. Instead, several signals are often integrated by one (or more) transcriptional regulator by a combination of mechanisms at the promoter.
Abstract
Bacteria use their genetic material with great effectiveness to make the right products in the correct amounts at the appropriate time. Studying bacterial transcription initiation in Escherichia coli has served as a model for understanding transcriptional control throughout all kingdoms of life. Every step in the pathway between gene and function is exploited to exercise this control, but for reasons of economy, it is plain that the key step to regulate is the initiation of RNA-transcript formation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ebright, R. H. RNA polymerase: structural similarities between bacterial RNA polymerase and eukaryotic RNA polymerase II. J. Mol. Biol. 304, 687–698 (2000).
Zhang, G. et al. Crystal structure of Thermus aquaticus core RNA polymerase at 3.3Å resolution. Cell 98, 811–824 (1999).
Fu, J. et al. Yeast RNA polymerase II at 5Å resolution. Cell 98, 799–810 (1999).
Korzheva, N. et al. A structural model for transcription elongation. Science 289, 619–625 (2000). A masterful article that explains how to apply intelligent chemistry to supplement structural data to produce a model for the bacterial transcription elongation complex.
Blatter, E. E., Ross, W., Tang, H., Gourse, R. L. & Ebright, R. H. Domain organisation of RNA-polymerase α-subunit: C-terminal 85 amino-acids constitute a domain capable of dimerisation and DNA-binding. Cell 78, 889–896 (1994).
Gourse, R. L., Ross, W. & Gaal T. UPs and downs in bacterial transcription initiation: the role of the α subunit of RNA polymerase in promoter recognition. Mol. Microbiol. 37, 687–695 (2000). A nicely written micro-review that summarizes our current understanding about the RNA polymerase α-subunit C-terminal domain.
Hampsey, M. Omega meets its match. Trends Genet. 17, 190–191 (2001).
Gross, C. et al. in Transcription. Cold Spring Harbor Symposia on Quantitative Biology, LXIII. 141–155 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York 1998). Recommended reading for University of Birmingham undergraduates! A gentle introduction to σ factors that quickly leads to outlining the present problems.
Wösten, M. M. S. M. Eubacterial sigma-factors. FEMS Microbiol. Rev. 22, 127–150 (1998).
Merrick, M. J. In a class of its own — the RNA polymerase sigma factor, σ54. Mol. Microbiol. 10, 903–909 (1993).
Campbell, E. A. et al. Structure of the bacterial RNA polymerase promoter specificity α subunit. Mol. Cell. 9, 527–539 (2002). Explains the structural basis for the organisation of σ factors.
Murakami, K. S., Masuda, S. & Darst, S. A. Structural basis of transcription initiation: RNA polymerase holoenzyme at 4Å resolution. Science 296, 1280–1284 (2002).
Murakami, K. S., Masuda, S., Campbell, E. A., Muzzin, O. & Darst, S. A. Structural basis of transcription initiation: an RNA polymerase holoenzyme–DNA complex. Science 296, 1285–1290 (2002). References 12 and 13 represent a quantum leap in our understanding of RNA polymerase and its interactions with promoters
Vassylyev, D. G. et al. Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 Å resolution. Nature 417, 712–719 (2002).
de Haseth, P. L., Zupancic, M. L. & Record, M. T. Jr. RNA polymerase–promoter interactions: the comings and goings of RNA polymerase. J. Bacteriol. 180, 3019–3025 (1998).
Busby, S. & Ebright R. H. Promoter structure, promoter recognition, and transcription activation in prokaryotes. Cell 79, 743–746 (1994).
Bown, J., Barne, K., Minchin, S. & Busby, S. in Nucleic Acids and Molecular Biology Vol. 11 (eds Lilley, D. & Eckstein, F.) 41–52 (Springer, New York, 1997).
Sanderson, A., Mitchell, J. E., Minchin, S. D. & Busby, S. J. Substitutions in the Escherichia coli RNA polymerase σ70 factor that affect recognition of extended −10 elements at promoters. FEBS Lett. 544, 199–205 (2003).
Ross, W., Ernst, A. & Gourse, R. L. Fine structure of E. coli RNA polymerase-promoter interactions: α subunit binding to the UP element minor groove. Genes Dev. 15, 491–506 (2001). A great illustration of how clever biochemistry can be used to discover basic information about the ways in which a protein recognizes its DNA target.
Tsujikawa, L., Tsodikov, O. V. & deHaseth, P. L. Interaction of RNA polymerase with forked DNA: evidence for two kinetically significant intermediates on the pathway to the final complex. Proc. Natl Acad. Sci. USA 99, 3493–3498 (2002).
Tomsic, M. et al. Different roles for basic and aromatic amino acids in conserved region 2 of Escherichia coli σ70 in the nucleation and maintenance of the single-stranded DNA bubble in open RNA polymerase-promoter complexes. J. Biol. Chem. 276, 31891–31896 (2001).
Ishihama, A. Functional modulation of Escherichia coli RNA polymerase. Annu. Rev. Microbiol. 54, 499–518 (2000).
Maeda, H., Fujita, N. & Ishihama A. Competition among seven Escherichia coli σ subunits: relative binding affinities to the core RNA polymerase. Nucleic Acids Res. 28, 3497–3503 (2000).
Blomfield, I. C. in Signals, Switches, Regulons, and Cascades (eds Hodgson, D. A. and Thomas, C. M.) 57–72 (Cambridge Univ. Press, Cambridge, UK, 2002).
Salgado, H. et al. RegulonDB (version 3.2): transcriptional regulation and operon organization in Escherichia coli K-12. Nucleic Acids Res. 29, 72–74 (2001).
Yura, T. & Nakahigashi, K. Regulation of the heat-shock response. Curr. Opin. Microbiol. 2, 153–158 (1999).
Raivio, T. L. & Silhavy, T. J. Periplasmic stress and ECF sigma factors. Annu. Rev. Microbiol. 55, 591–624 (2001).
Hughes, K. & Mathee, K. The anti-sigma factors. Annu. Rev. Microbiol. 52, 231–286 (1998).
Chatterji, D. & Ojha, A. K. Revisiting the stringent response, ppGpp and starvation signaling. Curr. Opin. Microbiol. 4, 160–165 (2001).
Barker, M. M., Gaal, T., Josaitis, C. A. & Gourse, R. L. Mechanism of regulation of transcription initiation by ppGpp. I. Effects of ppGpp on transcription initiation in vivo and in vitro. J. Mol. Biol. 305, 673–688 (2001).
Barker, M. M., Gaal, T. & Gourse, R. L. Mechanism of regulation of transcription initiation by ppGpp. II. Models for positive control based on properties of RNAP mutants and competition for RNAP. J. Mol. Biol. 305, 689–702 (2001).
Gaal, T., Bartlett, M. S., Ross, W., Turnbough, C. L. Jr & Gourse, R. L. Transcription regulation by initiating NTP concentration: rRNA synthesis in bacteria. Science 278, 2092–2097 (1997).
Schneider, D. A., Gaal, T. & Gourse, R. L. NTP-sensing by rRNA promoters in Escherichia coli is direct. Proc. Natl Acad. Sci. USA 99, 8602–8607 (2002).
Schneider, D. A., Ross, W. & Gourse, R. L. Control of rRNA expression in Escherichia coli. Curr. Opin. Microbiol. 6, 151–156 (2003). A nice summary of our present understanding of growth control and of transcription initiation.
Pérez-Rueda, E. & Collado-Vides, J. The repertoire of DNA-binding transcriptional regulators in Escherichia coli K-12. Nucleic Acids Res. 28, 1838–1847 (2000).
Babu, M. M. & Teichmann, S. A. Evolution of transcription factors and the gene regulatory network in Escherichia coli K-12. Nucleic Acids Res. 31, 1234–1244 (2003).
Martinez-Antonio, A. & Collado-Vides, J. Identifying global regulators in transcriptional regulatory networks in bacteria. Curr. Opin. Microbiol. 6, 482–489 (2003).
Müller-Hill, B. The Lac Operon: A Short History of a Genetic Paradigm. (Walter de Gruyter, New York, 1996). A beautifully crafted account of the lac system. Although written from a historical perspective, the text probes current issues and is overlaid with the author's personal philosophy.
Stock, A. M., Robinson, V. L. & Goudreau, P. N. Two-component signal transduction. Annu. Rev. Biochem. 69, 183–215 (2000).
Darwin, A. J. & Stewart, V. in Regulation of Gene Expression in E. coli. (eds Lin, E. C. C. & Lynch, A. S.) 343–359 (R. G. Landes, New York, 1996).
Demple, B. Redox signaling and gene control in the Escherichia coli soxRS oxidative stress regulon — a review. Gene 179, 53–57 (1996).
Plumbridge, J. Regulation of gene expression in the PTS in Escherichia coli: the role and interactions of Mlc. Curr. Opin. Microbiol. 5, 187–193 (2002).
Griffith, K. L., Shah, I. M., Myers, T. E., O'Neill, M. C. & Wolf, R. E. Jr. Evidence for 'pre-recruitment' as a new mechanism of transcription activation in Escherichia coli: the large excess of SoxS binding sites per cell relative to the number of SoxS molecules per cell. Biochem. Biophys. Res. Commun. 291, 979–986 (2002).
Martin, R. G., Gillette, W. K., Martin, N. I. & Rosner, J. L. Complex formation between activator and RNA polymerase as the basis for transcriptional activation by MarA and SoxS in Escherichia coli. Mol. Microbiol. 43, 355–370 (2002).
Ebright, R. H. Transcription activation at Class I CAP-dependent promoters. Mol. Microbiol. 8, 797–802 (1993).
Dove, S. L., Darst, S. A. & Hochschild, A. Region 4 of σ as a target for transcription regulation. Mol. Microbiol. 48, 863–874 (2003).
Nickels, B. E., Dove, S. L., Murakami, K. S., Darst, S. A. & Hochschild, A. Protein–protein and protein–DNA interactions of σ70 region 4 involved in transcription activation by λcI. J. Mol. Biol. 324, 17–34 (2002).
Busby, S. & Ebright, R. H. Transcription activation at Class II CAP-dependent promoters. Mol. Microbiol. 23, 853–859 (1997).
Sheridan, S. D., Benham, C. J. & Hatfield, G. W. Activation of gene expression by a novel DNA structural transmission mechanism that requires supercoiling-induced DNA duplex destabilization in an upstream activating sequence. J. Biol. Chem. 273, 21298–21308 (1998).
Sheridan, S. D., Opel, M. L. & Hatfield, G. W. Activation and repression of transcription initiation by a distant DNA structural transition. Mol. Microbiol. 40, 684–690 (2001). Rightly or wrongly, it is said that it is the exception that proves the rule — this article advocates the exceptional cases.
Brown, N. L., Stoyanov, J. V., Kidd, S. P. & Hobman, J. L. The MerR family of transcriptional regulators. FEMS Microbiol. Rev. 27, 145–163 (2003).
Heldwein, E. E. & Brennan, R. G. Crystal structure of the transcription activator BmrR bound to DNA and a drug. Nature 409, 378–382 (2001). Great crystallography, an interesting problem and an unexpected result!
Müller-Hill, B. Some repressors of bacterial transcription. Curr. Opin. Microbiol. 1, 145–151 (1998).
Choy, H. & Adhya, S. in Escherichia coli and Salmonella: Cellular and Molecular Biology (ed. Neidhardt, F. C.) 1287–1299 (ASM Press, Washington, 1996).
Shin, M. et al. Repression of deoP2 in Escherichia coli by CytR: conversion of a transcription activator into a repressor. EMBO J. 20, 5392–5399 (2001).
Valentin-Hansen, P., Sogaard-Andersen, L. & Pedersen, H. A flexible partnership: the CytR anti-activator and the cAMP-CRP activator protein, comrades in transcription control. Mol. Microbiol. 20, 461–466 (1996).
Azam, T. A. & Ishihama, A. Twelve species of the nucleoid-associated protein from Escherichia coli. J. Biol. Chem. 274, 33105–33113 (1999).
Schnetz, K. Silencing of Escherichia coli bgl promoter by flanking sequence elements. EMBO J. 14, 2545–50 (1995).
Jordi, B. J. A. M. & Higgins, C. F. The downstream regulatory element of the proU operon of Salmonella typhimurium inhibits open complex formation by RNA polymerase at a distance. J. Biol. Chem. 275, 12123–12128 (2000).
Petersen, C., Moller, L. B. & Valentin-Hansen, P. The cryptic adenine deaminase gene of Escherichia coli. Silencing by the nucleoid-associated DNA-binding protein, H-NS, and activation by insertion elements. J. Biol. Chem. 277, 31373–31380 (2002).
McLeod, S. M. & Johnson, R. C. Control of transcription by nucleoid proteins. Curr. Opin. Microbiol. 4, 152–159 (2001). A very clear summary of an interesting and topical subject.
Browning, D. F., Cole, J. A. & Busby, S. J. W. Suppression of FNR-dependent transcription activation at the Escherichia coli nir promoter by Fis, IHF and H-NS: modulation of transcription by a complex nucleo-protein assembly. Mol. Microbiol. 37, 1258–1269 (2000).
Gerstel, U., Park, C. & Romling, U. Complex regulation of csgD promoter activity by global regulatory proteins. Mol. Microbiol. 49, 639–654 (2003).
Dixon, R. in Signals, Switches, Regulons, and Cascades (eds Hodgson, D. A. & Thomas C. M.) 212–230 (Cambridge Univ. Press, UK, 2002).
Busby, S. & Kolb, A. in Regulation of Gene Expression in E. coli. (eds Lin, E. C. C. & Lynch, A. S.) 255–279 (R. G. Landes, New York, 1996).
Chahla, M., Wooll, J., Laue, T. M., Nguyen, N. & Senear, D. F. Role of protein-protein bridging interactions on cooperative assembly of DNA-bound CRP-CytR-CRP complex and regulation of the Escherichia coli CytR regulon. Biochemistry 42, 3812–3825 (2003).
Richet, E., Vidal-Ingigliardi, D. & Raibaud, O. A new mechanism for coactivation of transcription initiation: repositioning of an activator triggered by the binding of a second activator. Cell 66, 1185–1195 (1991).
Schroder, I., Darie, S. & Gunsalus, R. P. Activation of the Escherichia coli nitrate reductase (narGHJI) operon by NarL and Fnr requires integration host factor. J. Biol. Chem. 268, 771–774 (1993).
Joung, J. K., Koepp, D. M. & Hochschild, A. Synergistic activation of transcription by bacteriophage lambda cI protein and E. coli cAMP receptor protein. Science 265, 1863–1866 (1994).
Scott, S., Busby, S. & Beacham, I. Transcriptional coactivation at the ansB promoters: involvement of the activating regions of CRP and FNR when bound in tandem. Mol. Microbiol. 18, 521–532 (1995).
McLeod, S. M., Aiyar, S. E., Gourse, R. L. & Johnson, R. C. The C-terminal domains of the RNA polymerase σ subunits: contact site with Fis and localization during co-activation with CRP at the Escherichia coli proP P2 promoter. J. Mol. Biol. 316, 517–529 (2002).
Belyaeva, T., Rhodius, V., Webster, C. & Busby, S. Transcription activation at promoters carrying tandem DNA sites for the Escherichia coli cyclic AMP receptor protein: organisation of the RNA polymerase σ subunits. J. Mol. Biol. 277, 789–804 (1998).
Beatty, C., Browning, D., Busby, S. & Wolfe, A. CRP-dependent activation of the Escherichia coli acsP2 promoter by a synergistic Class III mechanism. J. Bacteriol. 185, 5148–5157 (2003).
Tebbutt, J., Rhodius, V., Webster, C. & Busby, S. Architectural requirements for optimal activation by tandem CRP molecules at a Class I CRP-dependent promoter. FEMS Lett. 210, 55–60 (2002).
Wade, J., Belyaeva, T., Hyde, E. & Busby, S. A simple mechanism for codependence on two activators at an E. coli promoter. EMBO J. 20, 7160–7167 (2001).
Wu, H-C., Tyson, K., Cole, J. & Busby, S. Regulation of the E. coli nir operon by two transcription factors: a new mechanism to account for co-dependence on two activators. Mol. Microbiol. 27, 493–505 (1998).
Browning, D. F., Beatty, C. M., Wolfe, A. J., Cole, J. A. & Busby, S. J. W. Independent regulation of the divergent Escherichia coli nrfA and acsP1 promoters by a nucleoprotein assembly at a shared regulatory region. Mol. Microbiol. 43, 687–701 (2002).
Eriksson, S., Lucchini, S., Thompson, A., Rhen, M. & Hinton, J. C. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol. Microbiol. 47, 103–118 (2003).
Reisner, A., Haagensen, J. A., Schembri, M. A., Zechner, E. L. & Molin, S. Development and maturation of Escherichia coli K-12 biofilms. Mol. Microbiol. 48, 933–946 (2003).
Burgess, R. R., Travers, A. A., Dunn, J. J. & Bautz, E. K. Factor stimulating transcription by RNA polymerase. Nature 221, 43–46 (1969).
Gruber, T. M. & Gross, C. A. Multiple sigma subunits and the partitioning of bacterial transcription space. Annu. Rev. Microbiol. 57, 441–466 (2003).
Jishage, M., Kvint, K., Shingler, V. & Nystrom, T. Regulation of sigma factor competition by the alarmone ppGpp. Genes Dev. 16, 1260–1270 (2002).
Helmann, J. D. Anti-sigma factors. Curr. Opin. Microbiol. 2, 135–141 (1999). An informative account of what is known about anti-sigma factors.
Hengge, R. & Bukau, B. Proteolysis in prokaryotes: protein quality control and regulatory principles. Mol. Microbiol. 49, 1451–1462 (2003).
Studemann, A. et al. Sequential recognition of two distinct sites in σS by the proteolytic targeting factor RssB and ClpX. EMBO J. 22, 4111–4120 (2003). Together with Reference 84, this paper describes a series of experiments that tackle a complicated problem with amazing directness. A paper for connoisseurs.
Buck, M., Gallegos, M.-T., Studholme, D. J., Guo, Y. & Gralla, J. D. The bacterial enhancer-dependent σ54 (σN) transcription factor. J. Bacteriol. 182, 4129–4136 (2000).
Acknowledgements
Work in the authors' laboratory is funded by the Wellcome Trust and the UK Biotechnology and Biological Sciences Research Council. We are grateful to the very many colleagues who have discussed their ideas with us, and we apologise to those whose work we have been unable to cite due to space limitations. We thank A. Barnard and G. Lloyd for critical scrutiny of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- CRAB-CLAW STRUCTURE
-
This is the name given to the structure that is probably common to all multi-subunit RNA polymerases, in which the two largest subunits form a cleft that contains the enzyme active site.
- LINKER
-
In the context of a protein, a linker is a short stretch of amino acids that joins two separately folding domains. Many linkers have a flexible structure that allows the adjacent domains to adopt different juxtapositions with respect to each other.
- SIGMA FACTOR
-
The subunit of RNA polymerase holoenzyme that is required for promoter sequence recognition and ability to initiate transcription.
- OPEN COMPLEX
-
For transcription, the two strands of the DNA duplex must be unwound locally. An open complex is formed when RNA polymerase binds at a promoter, and the duplex around the transcription start is unwound.
- UP ELEMENT
-
This is a DNA sequence element found at some promoters that increases promoter activity by providing a point of contact for the RNA polymerase α subunit C-terminal domains.
- ISOMERIZATION
-
Describes the step in which the DNA segment, in the RNA polymerase-promoter complex, is unwound.
- ANTI-SIGMA FACTORS
-
A negative transcriptional regulator that acts by binding to a sigma factor and preventing its activity. An anti-anti-sigma factor, in turn, counteracts the action of an anti-sigma factor.
- RESPONSE REGULATORS
-
Usually bacterial gene-regulatory proteins that control gene expression in response to external signals. Most response regulators consist of two domains: a DNA-binding domain and a regulatory domain, the activity of which is modulated (indirectly) by the external signal.
- SENSOR KINASE
-
Transmits the external signal to the response regulator.
- SUPERCOILING
-
Describes a state of the DNA in which its conformation deviates from the well-known Watson-Crick double helix, leading to its compaction and favouring local DNA unwinding.
Rights and permissions
About this article
Cite this article
Browning, D., Busby, S. The regulation of bacterial transcription initiation. Nat Rev Microbiol 2, 57–65 (2004). https://doi.org/10.1038/nrmicro787
Issue Date:
DOI: https://doi.org/10.1038/nrmicro787
This article is cited by
-
Identification and characterization of a strong constitutive promoter stnYp for activating biosynthetic genes and producing natural products in streptomyces
Microbial Cell Factories (2023)
-
Newly isolated Lactobacillus paracasei strain modulates lung immunity and improves the capacity to cope with influenza virus infection
Microbiome (2023)
-
Programmable synthetic biomolecular condensates for cellular control
Nature Chemical Biology (2023)
-
Atomic force microscopy-based approaches for single-molecule investigation of nucleic acid–protein complexes
Biophysical Reviews (2023)
-
Genome-scale analysis of genetic regulatory elements in Streptomyces avermitilis MA-4680 using transcript boundary information
BMC Genomics (2022)