Abstract
The recent association between Zika virus (ZIKV) infection during pregnancy and fetal microcephaly has led to a renewed interest in the mechanisms by which vertically transmitted microorganisms reach the fetus and cause congenital disease. In this Opinion article, we provide an overview of the structure and cellular composition of the human placenta and of the mechanisms by which traditional 'TORCH' pathogens (Toxoplasma gondii, other, rubella virus, cytomegalovirus and herpes simplex virus) access the fetal compartment. Based on our current understanding of ZIKV pathogenesis and the developmental defects that are caused by fetal ZIKV infection, ZIKV should be considered a TORCH pathogen and future research and public health measures should be planned and implemented accordingly.
Similar content being viewed by others
Main
Zika virus (ZIKV), a member of the Flaviviridae family of RNA viruses, was first isolated in the Zika forest in Uganda in 1947 (Refs 1,2). Prior to 2007, human ZIKV infections were only reported in Africa and Asia, confirmed cases were rare and only mild symptoms, such as rash, fever, headaches, arthralgia and conjunctivitis, were described3,4,5. When ZIKV was first detected in Brazil in early 2015 it came as a surprise, because this obscure mosquito-borne virus had not previously been reported in the Western Hemisphere6,7. However, ZIKV had been spreading throughout Oceania in the prior decade and subsequent genetic analysis showed that ZIKV spread to Brazil from the South Pacific8. Initial case reports in Brazil were consistent with the fairly benign disease that was reported in previous outbreaks of ZIKV. This changed in the autumn of 2015 when physicians in the north-eastern states of Brazil reported a surge in cases of microcephaly and other congenital abnormalities that coincided with the spread of ZIKV in this region9. Since then, it has become evident that ZIKV infection during pregnancy is associated with fetal microcephaly9,10,11,12,13,14,15,16. Microcephaly is a severe disorder of fetal brain development that results in a head size that is smaller than normal and is often accompanied by cognitive and physical delays. ZIKV-associated microcephaly, in particular, involves a 'fetal brain disruption sequence' (Refs 13,17,18), which includes growth arrest of the cerebrum, partial collapse of the skull, and distinct folds from redundant scalp skin. ZIKV infections in pregnant women have also been associated with other fetal disorders such as placental insufficiency and fetal growth restriction, ocular disorders, other central nervous system anomalies and even fetal demise19,20,21. These disorders, which together with microcephaly, have been called 'congenital Zika syndrome' (Refs 13,16,21), are reminiscent of those that result from congenital infections caused by 'TORCH' pathogens (this acronym is widely used in the clinic and stands for Toxoplasma gondii, other, rubella virus, cytomegalovirus (CMV) and herpes simplex virus (HSV)). Remarkably, ZIKV can induce fetal damage well beyond the first trimester, as infections even late during pregnancy can result in fetal disease and/or adverse pregnancy outcomes16,19.
The emergence of congenital Zika syndrome has led to a renewed interest in the mechanisms by which vertically transmitted microorganisms reach the fetal compartment. In this Opinion article, we discuss the structure of the human placenta, the mechanisms by which other microorganisms that are associated with congenital disease breach the placental barrier and the current models to study ZIKV pathogenesis both in vitro and in vivo. What we know about ZIKV suggests that it should be classified as a TORCH pathogen, so that the experience with traditional TORCH pathogens can inform future research and public health priorities regarding ZIKV.
The placental barrier
To reach the human fetus, ZIKV must overcome the barrier presented by the placenta, which develops within days of conception and is indispensable for pregnancy.
In the early stages of pregnancy, the human placenta is responsible for anchoring the blastocyst to the maternal endometrium and for establishing the circulatory system that will feed maternal blood directly into the intervillous space (IVS). The IVS is the fluid-filled cavity that bathes the villous tree surfaces of the human placenta, which are formed by syncytiotrophoblasts (SYNs; Box 1). Owing to their direct contact with maternal blood, SYNs are crucial for protecting the fetus from pathogens. The SYN layer forms a protective barrier even at the earliest stages of pregnancy, with a complete layer of SYNs surrounding the embryo by the end of implantation (∼7 days). Defects, even small ones, at any stage of the formation or development of the placenta can lead to miscarriage. Once the uteroplacental circulatory system is fully established, which occurs near the end of the first trimester (∼12 weeks), the placenta is the sole barrier that prevents microorganisms in the maternal blood from accessing the fetal compartment. Earlier, the IVS does not contain maternal blood and is instead filled with clear fluid that is produced by maternal and/or fetal cells22,23,24. Thus, mechanisms of vertical transmission probably differ between the early and later (>12 weeks) stages of human pregnancy.
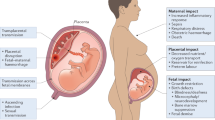
Vertical transmission of pathogens from mother to fetus can occur by several routes, including: infection of endothelial cells in the maternal microvasculature and spread to invasive extravillous trophoblasts (EVTs), which are the cells that anchor the villous trees to the uterine wall; trafficking of infected maternal immune cells across the placental barrier; paracellular or transcellular transport (for example, immunoglobulin-mediated transcytosis) from maternal blood across the villous trees and into the fetal capillaries; damage to the villous tree and breaks in the SYN layer; and/or transvaginal ascending infection (Fig. 1). The ability of ZIKV to infect and damage developing fetuses implies that the virus can cross and/or bypass the placental barrier. Other flaviviruses, including important human pathogens such as dengue virus (DENV), are not generally associated with vertical transmission or congenital disorders, which suggests that this mechanism may be specific to ZIKV.
There are several pathways by which Zika virus (ZIKV), and other TORCH (Toxoplasma gondii, other, rubella virus, cytomegalovirus (CMV), herpes simplex virus (HSV)) pathogens, might be vertically transmitted. Pathogens might invade by several routes and these routes can vary depending on the gestational stage. The precise routes that are used by different pathogens remain largely unknown. Ascending infection with HSV and possibly by Treponema pallidum might expose the placenta to these pathogens. In the case of both T. gondii and Listeria monocytogenes, studies using human first trimester explants suggest that extravillous trophoblasts (EVTs) are the 'Achilles heel' that enables the delivery of these pathogens to the fetal compartment, without infection of syncytiotrophoblasts (SYNs). The mechanisms by which rubella virus and CMV cross the placental barrier are less clear, although CMV might be transported across the trophoblast layers by antibody-dependent transcytosis without replication in the trophoblast layers. It is unknown how ZIKV crosses the placental barrier. Potential routes of vertical transmission include: breaks in the SYN layer (which could result from maternal immune signals), exposing the more susceptible cells of the villous core; direct infection of the SYN layer; bypassing the SYN layer through non-replicative routes (such as antibody-mediated transcytosis); infection of EVTs or other more permissive placental cell types, most probably through the maternal microvasculature and/or decidua; delivery to the fetus and/or villous core through maternal cells (most likely those of immune origin); and/or ascending intravaginal infection. IgG, immunoglobulin G.
TORCH pathogens
Despite the robust protection it provides, some microorganisms can overcome the placental barrier. The term 'TORCH' is used to designate microorganisms that are associated with known congenital disease and stands for T. gondii, other (Listeria monocytogenes, Treponema pallidum, varicella zoster virus (VZV), human immunodeficiency virus (HIV), enteroviruses and parvovirus B19), rubella virus, CMV, and HSV-1 and HSV-2 (Ref. 25). The congenital disorders that are induced by TORCH pathogens can be devastating. During a rubella epidemic in the United States in the mid-1960s ∼20,000 infants were born with congenital rubella syndrome and ∼2000 infants died as a result of congenital infection26. Although not typically classified as a TORCH pathogen, placental infections that are caused by Plasmodium spp., the parasites that are responsible for malaria, are often associated with severe harm to the developing fetus (most commonly growth restriction and/or preterm birth)27. Malaria continues to be a source of devastating illness in pregnant women and is responsible for over 200,000 neonatal deaths each year27.
The similarities between the congenital disorders that are induced by TORCH pathogens and ZIKV are remarkable, particularly with respect to their neurotropism. Similar to ZIKV, fetal infection by T. gondii, rubella virus, CMV, VZV, and other pathogens, are associated with microcephaly, although the fetal brain disruption sequence seems to be characteristic of ZIKV-associated microcephaly13,16. Fetal ZIKV infection produces a cytopathic effect and a lack of inflammation in brain histopathology that are reminiscent of congenital rubella syndrome28. The specific congenital malformations that are induced by TORCH pathogens, and probably ZIKV, depend on the gestational age at fetal infection. For example, in the case of rubella virus, infection in the first trimester (<12 weeks) is associated with high rates of miscarriage and with the most severe congenital malformations. As pregnancy progresses, the risk of congenital malformations that result from rubella virus infection decreases and becomes very low during and after the second trimester. Similarly, although the risk of vertical transmission of CMV is highest in the third trimester, the risk of fetal malformations caused by CMV infections is greatest in the first trimester, probably resulting from the key fetal developmental processes that occur at this stage of gestation.
It seems likely that the first trimester15 will represent the gestational period when the fetus is most sensitive to ZIKV- associated microcephaly, and a recent study found no adverse outcomes among women who were infected with ZIKV during their third trimester29. However, other reports found various adverse outcomes including placental insufficiency, intrauterine growth restriction, cerebral calcifications as well as microcephaly following ZIKV infections at various stages of gestation16,19. In addition, it is important to note that ZIKV-induced congenital abnormalities, such as neurological and ocular disorders, can present without microcephaly16,30. Most alarmingly, fetal demise has been reported in women who were infected as late as 25 weeks and 32 weeks of gestation, which suggests that ZIKV can have devastating fetal consequences at various gestational ages19. Furthermore, ZIKV-associated microcephaly has been reported even in mothers who have asymptomatic ZIKV infection16,29, which suggests that the severity of maternal illness may not predict fetal outcomes. Prospective longitudinal studies are required to better define the manifestations of congenital ZIKV syndrome, identify risk factors that contribute to adverse outcomes and to determine the effect of ZIKV infection at different stages of gestation. Such efforts are already underway: the United States National Institutes of Health and Brazilian Fundacao Oswaldo Cruz-Fiocruz recently initiated a large study focused on Puerto Rico, Brazil and Colombia, and other studies are planned and in progress31.
Mechanisms of vertical transmission
The mechanisms of vertical transmission of TORCH pathogens remain largely unknown. For both T. gondii and L. monocytogenes, studies in first trimester human explant models indicate that the SYN layer forms an effective barrier to the passage of both pathogens32,33. Given the broad antiviral resistance of primary SYNs isolated from full-term placentas34,35,36, it seems likely that viruses access the fetus through routes that do not involve replication in the SYN layer, at least at later stages of pregnancy. EVTs may be an entry portal for microorganisms that enables them to bypass the SYN barrier32,33,37, as suggested in a recent study of ZIKV that used first trimester explants and second trimester primary cells38. However, it remains unclear how pathogens reach these cells, as EVTs are buried in the decidua; possibly these cells are exposed to maternal blood during vascularization and/or contact infected maternal immune cells. Although SYNs that are isolated from full-term placentas and SYNs that are exposed to virus from first trimester explants resist ZIKV infection36,38, it is possible that ZIKV replicates in SYNs at very early stages of gestation, or that the virus targets other placental cells, such as EVTs. ZIKV RNA and antigens have been detected in the placentas of fetuses with microcephaly28,39, and a mouse model of ZIKV vertical transmission found high viral loads in the placenta, as well as viral genomes and viral particles in placental trophoblasts40 (Table 1). These findings, coupled with more recent work that used first trimester human placental explants38, are consistent with a model in which ZIKV from the maternal circulation infects EVTs and then spreads to the fetus. However, the anatomical and immunological differences between the mouse and human placentas may limit the direct applicability of results from mouse studies to humans41,42 (Box 1). In the same mouse model, ZIKV triggered apoptosis and vascular damage in the placenta, which could contribute to vertical transmission by directly damaging the trophoblast layers. Fetal-derived macrophages (Hofbauer cells) are susceptible to ZIKV infection and might be important for vertical transmission38,43. Furthermore, ZIKV antigens were detected in Hofbauer cells from fetuses with microcephaly28. However, given that Hofbauer cells are not in direct contact with the maternal circulation and are primarily located close to fetal capillaries, pathogens still require a mechanism to cross the SYN layer of the placenta before accessing these cells. A recent comprehensive study that used first trimester human placental explants found that several placental cell types might be targeted by ZIKV early in gestation, including both cytotrophoblasts (CYTs) and EVTs, which suggests that there may be several routes for ZIKV vertical transmission38. These could involve viral replication in select placental cell populations, and/or non-replicative routes such as in trafficking immune cells or by transcytosis. Furthermore, the maternal immune response might damage the placenta, which may allow viral passage. Infected maternal cells could deliver ZIKV to the decidua, which may lead to the infection of EVTs. Transmigration of infected maternal immune cells could also deliver ZIKV to the fetus, similarly to the transcytosis of fetal cells into the maternal circulation44. The transfer of maternal immunoglobulin G (IgG) is crucial to protect the developing fetus, particularly during the stages when the fetus is most sensitive to viral infections. However, in some cases, this process may expose the fetus to pathogens; for example, maternal IgG antibodies might carry CMV across the SYN layer through the neonatal Fc receptor for IgG (FcRn)45. Among flaviviruses, the ability of antibodies to facilitate viral infection of FcR-bearing cells is well established for DENV, a process termed antibody-dependent enhancement46,47. There are four serotypes of DENV, and secondary infection with a heterologous serotype can result in exacerbated disease, as cross-reactive non-neutralizing antibodies promote FcR-mediated infection of myeloid cells. Furthermore, severe disease is also seen during primary DENV infections in infants, as infection is enhanced by circulating maternal antibodies48. Given the emergence of ZIKV in areas with high DENV seroprevalence, and the substantial antigenic cross-reactivity between ZIKV and DENV49,50,51,52,53, it is plausible that maternal DENV antibodies could contribute to ZIKV placental invasion through FcRn-mediated transcytosis. However, it is worth noting that transplacental delivery of maternal IgG is quite low during the first trimester (before the establishment of a haemochorial placenta), with levels increasing in the second and third trimesters54,55,56,57,58. In vitro studies have demonstrated that DENV immune sera and DENV monoclonal antibodies can enhance ZIKV infection in cell culture, but the importance of this in vivo, at physiological antibody concentrations, and in placental cells specifically, remains to be determined50,51. Finally, ZIKV RNA has been detected in the female genital tract in humans and in rhesus macaques59,60, which raises the possibility that ZIKV could access the fetal compartment by transvaginal ascending infection, in addition to transplacental haematogenous spread.
Zika virus pathogenesis
As almost no laboratory research was carried out on ZIKV before the current epidemic, the pathogenic mechanisms of ZIKV are only beginning to be understood (Fig. 2). Aspects of ZIKV disease in humans, such as teratogenicity and sexual transmission, are unexpected based on extensive experience with related flaviviruses, which highlights the need to better understand this emerging pathogen2.
After crossing the placental barrier, Zika virus (ZIKV) infection can have devastating consequences for the developing fetus. Of note, unlike many TORCH (Toxoplasma gondii, other, rubella virus, cytomegalovirus (CMV) and herpes simplex virus (HSV)) pathogens, some evidence suggests that ZIKV can induce fetal disease and/or other adverse pregnancy outcomes throughout pregnancy. Potential adverse outcomes of fetal ZIKV infection include microcephaly, hydranencephaly, growth restriction, ocular abnormalities and death, whereas the virus causes mild or no symptoms in the pregnant mother. In addition, it is important to note that adverse outcomes due to in utero infections with some TORCH pathogens do not present until months or years after birth. Human organoid and mouse models support the destructive nature of ZIKV infections in the fetal brain.
The mechanisms that enable ZIKV to cross the placenta and cause fetal damage are of particular interest and importance. It remains unclear how ZIKV gains access to the fetal compartment, although possible mechanisms are discussed above. The full range of fetal tissues that are targeted by ZIKV remains to be determined, but ZIKV antigens and genomes were detected in the brains of infants and fetuses with microcephaly, without being detectable in other organs28,39,61. ZIKV infects neuroprogenitor cells in culture62,63, which is consistent with damage to the developing brain, although the outcome may depend on the gestational age when infection occurs. In vitro models using human stem cell-derived neural progenitors (Table 1) have confirmed the destructive nature of ZIKV infection in neural cells62,64,65,66,67. Such neurotropism is more characteristic of encephalitic flaviviruses (for example, West Nile virus and Japanese encephalitis virus) than of viruses such as DENV, to which ZIKV is more closely related. Although ZIKV typically causes an acute infection with viraemia cleared within one week, there is evidence of persistent maternal viraemia in the presence of a fetus infected with ZIKV39 and similar persistence was observed in pregnant rhesus macaques59. It is unclear whether this results from persistent viral shedding from the fetus or is a consequence of changes in the maternal immune system during pregnancy.
New small-animal models have been developed to study ZIKV infection in vivo (Table 1). These models recapitulate many aspects of ZIKV-induced disease in humans, such as infection of the brain, testes and fetus40,67,68,69,70,71. Recent reports demonstrate transplacental transmission of ZIKV in pregnant mice and associated fetal abnormalities including microcephaly, placental insufficiency, intrauterine growth restriction and fetal demise, all of which are relevant to congenital ZIKV infection in humans40,67,72. Mouse models of ZIKV pathogenesis and vertical transmission are crucial for understanding ZIKV pathogenesis and for testing candidate vaccines and antivirals. However, differences in anatomy and immune mechanisms between mouse and human placentas may limit the direct applicability of findings in mouse models to human disease73 (Box 1). For example, human SYNs that are isolated from full-term placentas constitutively produce interferon-λ1 (IFNλ1; also known as type III interferon or interleukin-29 (IL-29)), which protects cells from ZIKV infection in both a paracrine and autocrine manner36. However, mice do not express IFNλ1(Ref. 74); thus, the role of IFNλ in the protection of the murine placenta may be distinct from that in humans. Moreover, most existing mouse models of ZIKV pathogenesis use mice that lack the type I interferon receptor (for example, Ifnar1−/− mice) and thus have severely impaired antiviral responses68,69,70,71. Mice that have intact IFNα and IFNβ signalling show minimal viraemia and no disease signs or lethality after ZIKV infection. By contrast, transplacental transmission of ZIKV was demonstrated in Ifnar1−/− dams that were mated with wild-type sires, such that the affected fetuses and placentas would have intact IFNα and IFNβ signalling (Ifnar1+/−)40. Vertical transmission has also been demonstrated in wild-type SJL and C57Bl/6 mice67,72, although this required higher infection doses; ablated maternal IFNα and IFNβ signalling and high infection doses probably increase maternal viraemia to the level that is required for transplacental transmission.
Other groups have used direct intrauterine injection of mice to study the fetal pathogenesis of ZIKV72,75; however, this system does not model transplacental transmission from a viraemic mother. ZIKV infection in non-human primates, including rhesus macaques, mimics many aspects of human disease and, importantly, immune competent animals develop signs of disease1,59,76. Furthermore, the macaque placenta is similar to the human placenta and so serves as a better model of congenital infection than mice. Ongoing studies of ZIKV infection in pregnant rhesus macaques are likely to provide additional insight into the mechanisms of ZIKV transplacental transmission and teratogenicity59. In one study, pregnant mice that were infected with DENV did not show transplacental transmission and fetal damage as seen with ZIKV infection40. However, most animal and cell culture studies of ZIKV transplacental transmission and fetal neuropathogenesis have not directly compared ZIKV infection with related viruses, such as DENV, that are not generally associated with congenital infections in humans. Furthermore, congenital infection has only been apparent during the current ZIKV epidemic in the Americas; it is not known whether historical isolates of ZIKV, or contemporary isolates from different sources, share this property. Thus, the properties that determine ZIKV teratogenicity in humans, and how faithfully pathogenesis is recapitulated in current models, remain unclear.
Is ZIKV a TORCH pathogen?
The association between ZIKV and fetal microcephaly was unexpected, as flaviviruses are not generally associated with congenital infection. For comparison, maternal DENV infection during pregnancy is associated with adverse outcomes, including preterm birth and low birth weight77, which may be manifestations of maternal disease. However, there is no evidence of birth defects caused by DENV, despite millions of infections worldwide each year78,79. Although many unanswered questions remain, a growing body of clinical, epidemiological and molecular evidence supports a causal role for congenital ZIKV infection in the development of microcephaly13. The present evidence includes a spatiotemporal association between cases of microcephaly and ZIKV infections9; case–control studies of ZIKV-infected pregnant women in Brazil19; the detection of infectious ZIKV and complete ZIKV genomes in tissues of affected infants and fetuses14,39,61; cell culture and animal model studies that demonstrate ZIKV infection in relevant cells and tissues40,59,64,67,72; and a lack of other plausible explanations for the observed increase in cases of microcephaly in north-eastern Brazil. Although the emergence of ZIKV in Brazil coincided with an increase in cases of microcephaly, the total number of cases and the exact increase over baseline levels remain to be determined16. Ongoing research will provide a clearer picture of the rate at which maternal ZIKV infection results in transplacental infection and fetal damage, as well as how that risk changes during the course of gestation. Understanding these risks is crucial for pregnant women to make informed decisions and for the public health response to ZIKV.
The available evidence suggests that ZIKV should be considered a new member of the TORCH family of pathogens and the expanding category of 'other' invites a new acronym to describe teratogenic pathogens. For example, it has been proposed that the term TORCH be amended to 'TORCHES' or 'STORCH' to directly include syphilis, which is caused by T. pallidum80. Given the severe congenital anomalies that are associated with ZIKV, it may be appropriate to more directly include ZIKV in the acronym, such as using TORCHZ or another abbreviation. Unlike other TORCH pathogens, which are generally transmitted through person-to-person contact or through contaminated food, ZIKV typically is mosquito-borne and therefore requires substantially different public health control measures than other TORCH pathogens (Box 2). ZIKV can also be sexually transmitted81,82,83,84,85,86. Some evidence suggests that men shed infectious ZIKV in semen for weeks or months after the acute infection is resolved82,87, potentially acting as long-term transmission reservoirs. ZIKV RNA has been detected in the female reproductive tract37, although, to date, there has been a single report of female-to-male sexual transmission86 compared with dozens of reported cases from men. The contribution of sexual transmission compared with mosquito-borne transmission to outbreaks of ZIKV is unclear. A higher incidence of ZIKV infection has been reported in women than in men29,88, which could be caused by male-to-female sexual transmission augmenting mosquito-borne transmission to both sexes. However, other explanations include enhanced ZIKV awareness and surveillance among women owing to its association with birth defects; a greater willingness of women to seek medical care; an innate susceptibility of women due to immunological or other factors; and/or greater exposure to the anthropophilic domestic mosquitoes that transmit ZIKV. Furthermore, the role of sexual transmission for ZIKV congenital disease is unclear; if the virus reaches the fetal compartment through haematogenous spread, the initial route by which the virus infects the mother may not matter. Many questions remain regarding the teratogenic mechanisms of ZIKV, and experience with other TORCH pathogens should inform studies of the risk factors, transmission mechanisms and pathological effects of ZIKV.
Microcephaly was the first congenital abnormality that was associated with ZIKV infection, probably owing to its dramatic presentation9. However, similar to other TORCH pathogens, ZIKV probably causes a range of developmental abnormalities. The characteristics of congenital Zika syndrome are beginning to be described and include microcephaly, lissencephaly, ventriculomegaly, hydranencephaly, ocular abnormalities, placental insufficiency and fetal loss13,16,21,28. The long-term manifestations of TORCH infections may not be apparent at birth and often are diagnosed as the child develops. It will take time before the full range of ZIKV-associated birth defects becomes apparent, especially for more subtle effects such as hearing loss, cognitive and behavioural impairments, or other complications.
The available evidence indicates that ZIKV infection during pregnancy can result in transplacental transmission and fetal damage. However, we do not yet know whether ZIKV infection is sufficient for these effects, or whether additional factors contribute. No such cofactors that potentiate ZIKV teratogenicity have been identified, but these could include environmental factors, virus strain differences, co-infections, maternal immune responses and/or host genetics. It is unclear why ZIKV has been associated with birth defects during the current epidemic in Latin America, but not during historical outbreaks. The simplest explanation is that this ZIKV outbreak is vastly larger than previous ones: millions of people are estimated to have been infected in Latin America, compared with ∼100,000 in French Polynesia in 2013–2014, ∼5,000 in Micronesia in 2007 and only a handful of human cases reported in prior decades88,89. However, we know very little about the viral, host and environmental factors that contribute to ZIKV pathogenesis and additional studies are required.
Final conclusions
Many groups are focused on developing vaccines and antivirals to combat ZIKV, but a challenge is that these interventions must target pregnant women and/or couples who are planning to conceive (Box 2). The difficulty of testing new (and old) medications in pregnant women is a general problem; the reluctance to pursue such studies has resulted in a dearth of information regarding drug safety and efficacy during pregnancy, and is an obstacle to adequate prenatal care. Evaluation in pregnant women will be a crucial component of new ZIKV interventions and the development of innovative approaches to conduct these studies ethically may benefit maternal health more generally. There are currently very few effective treatments to prevent the vertical transmission of viral infections during pregnancy, although some antiviral therapies that reduce viral burden in the mother, such as antiretroviral therapies in HIV-positive women, can reduce viral loads to lessen the risk of vertical transmission90,91. Developing new therapeutic strategies to prevent vertical transmission of ZIKV and other TORCH pathogens will require a better understanding of the mechanisms that are used by these microorganisms to cross the placenta and cause fetal disease.
The long-term picture of ZIKV in the Western Hemisphere is unclear. Model-based predictions suggest that more than 1.5 million women may be at risk of contracting ZIKV during pregnancy and that in total ∼90 million individuals are at risk of infection during the current epidemic92. The unprecedented size of the current epidemic is probably a consequence of the emergence of the virus in an immunologically naive population, and the incidence of ZIKV infection is likely to decline in the presence of substantial population immunity. There is no evidence that prior ZIKV infection affects future pregnancies, thus the rates of microcephaly and other ZIKV-associated birth defects may decrease if most women are exposed to ZIKV before reaching child-bearing age. However, experience in Latin America with DENV and chikungunya virus, which are spread by the same mosquito vectors as ZIKV, suggests that continued ZIKV outbreaks and adult infections are likely. This highlights the need for a vaccine to ensure that women of child-bearing age are immune to ZIKV, much as routine immunization with the measles–mumps–rubella vaccine has eliminated congenital rubella syndrome from the Americas. However, amid all of the attention that is focused on ZIKV-associated birth defects, it is worth remembering that each year there are more than ∼100,000 cases of congenital rubella syndrome, ∼200,000 cases of congenital toxoplasmosis and ∼30,000 cases of congenital CMV worldwide93,94,95. The ongoing outbreak of ZIKV highlights the need not only to better understand how ZIKV and other TORCH pathogens breach the placental barrier and induce congenital disease, but also the urgent need to assess the safety and efficacy of antimicrobial therapeutics in pregnant women.
References
Dick, G. W., Kitchen, S. F. & Haddow, A. J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 46, 509–520 (1952).
Lazear, H. M. & Diamond, M. S. Zika virus: new clinical syndromes and its emergence in the Western hemisphere. J. Virol. 90, 4864–4875 (2016).
Fauci, A. S. & Morens, D. M. Zika virus in the Americas — yet another arbovirus threat. N. Engl. J. Med. 374, 601–604 (2016).
Simpson, D. I. Zika virus infection in man. Trans. R. Soc. Trop. Med. Hyg. 58, 335–338 (1964).
Bearcroft, W. G. Zika virus infection experimentally induced in a human volunteer. Trans. R. Soc. Trop. Med. Hyg. 50, 442–448 (1956).
Zanluca, C. et al. First report of autochthonous transmission of Zika virus in Brazil. Mem. Inst. Oswaldo Cruz 110, 569–572 (2015).
Campos, G. S., Bandeira, A. C. & Sardi, S. I. Zika virus outbreak, Bahia, Brazil. Emerg. Infect. Dis. 21, 1885–1886 (2015).
Faria, N. R. et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science 352, 345–349 (2016).
Schuler-Faccini, L. et al. Possible association between Zika virus infection and microcephaly — Brazil, 2015. MMWR. Morb. Mortal. Wkly Rep. 65, 59–62 (2016).
Cauchemez, S. et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet 387, 2125–2132 (2016).
Oliveira Melo, A. S. et al. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet. Gynecol. 47, 6–7 (2016).
Ventura, C. V., Maia, M., Bravo-Filho, V., Gois, A. L. & Belfort, R. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet 387, 228 (2016).
Rasmussen, S. A., Jamieson, D. J., Honein, M. A. & Petersen, L. R. Zika virus and birth defects — reviewing the evidence for causality. N. Engl. J. Med. 374, 1981–1987 (2016).
Sarno, M. et al. Zika virus infection and stillbirths: a case of hydrops fetalis, hydranencephaly and fetal demise. PLoS Negl. Trop. Dis. 10, e0004517 (2016).
Johansson, M. A., Mier-y- Teran-Romero, L., Reefhuis, J., Gilboa, S. M. & Hills, S. L. Zika and the risk of microcephaly. N. Engl. J. Med. 375, 1–4 (2016).
Franca, G. V. et al. Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet http://dx.doi.org/10.1016/S0140-6736(16)30902-3 (2016).
Corona-Rivera, J. R. et al. Report and review of the fetal brain disruption sequence. Eur. J. Pediatr. 160, 664–667 (2001).
Petersen, E. E. et al. Update: interim guidance for health care providers caring for women of reproductive age with possible Zika virus exposure — United States, 2016. MMWR. Morb. Mortal. Wkly Rep. 65, 315–322 (2016).
Brasil, P. et al. Zika virus infection in pregnant women in Rio de Janeiro — preliminary report. N. Engl. J. Med. http://dx.doi.org/10.1056/NEJMoa1602412 (2016).
Ventura, C. V. et al. Ophthalmological findings in infants with microcephaly and presumable intra-uterus Zika virus infection. Arq. Bras. Oftalmol. 79, 1–3 (2016).
Chan, J. F., Choi, G. K., Yip, C. C., Cheng, V. C. & Yuen, K. Y. Zika fever and congenital Zika syndrome: An unexpected emerging arboviral disease. J. Infect. 72, 507–524 (2016).
Hustin, J. & Schaaps, J. P. Echographic [corrected] and anatomic studies of the maternotrophoblastic border during the first trimester of pregnancy. Am. J. Obstet. Gynecol. 157, 162–168 (1987).
Foidart, J. M., Hustin, J., Dubois, M. & Schaaps, J. P. The human placenta becomes haemochorial at the 13th week of pregnancy. Int. J. Dev. Biol. 36, 451–453 (1992).
Burton, G. J., Jauniaux, E. & Watson, A. L. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd collection revisited. Am. J. Obstet. Gynecol. 181, 718–724 (1999).
Nahmias, A. J. et al. Perinatal risk associated with maternal genital herpes simplex virus infection. Am. J. Obstet. Gynecol. 110, 825–837 (1971).
Plotkin, S. A. Rubella eradication. Vaccine 19, 3311–3319 (2001).
Schantz-Dunn, J. & Nour, N. M. Malaria and pregnancy: a global health perspective. Rev. Obstet. Gynecol. 2, 186–192 (2009).
Martines, R. B. et al. Pathology of congenital Zika syndrome in Brazil: a case series. Lancet http://dx.doi.org/10.1016/S0140-6736(16)30883-2 (2016).
Pacheco, O. et al. Zika virus disease in Colombia — preliminary report. N. Engl. J. Med. http://dx.doi.org/10.1056/NEJMoa1604037 (2016).
Ventura, C. V., Maia, M., Dias, N., Ventura, L. O. & Belfort, R. Zika: neurological and ocular findings in infant without microcephaly. Lancet 387, 2502 (2016).
US National Institutes of Health. NIH launches large study of pregnant women in areas affected by Zika virus. NIH https://www.nih.gov/news-events/news-releases/nih-launches-large-study-pregnant-women-areas-affected-zika-virus (2016).
Robbins, J. R., Skrzypczynska, K. M., Zeldovich, V. B., Kapidzic, M. & Bakardjiev, A. I. Placental syncytiotrophoblast constitutes a major barrier to vertical transmission of Listeria monocytogenes. PLoS Pathog. 6, e1000732 (2010).
Robbins, J. R., Zeldovich, V. B., Poukchanski, A., Boothroyd, J. C. & Bakardjiev, A. I. Tissue barriers of the human placenta to infection with Toxoplasma gondii. Infect. Immun. 80, 418–428 (2012).
Bayer, A. et al. Human trophoblasts confer resistance to viruses implicated in perinatal infection. Am. J. Obstet. Gynecol. 212, 71 (2015).
Delorme-Axford, E. et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc. Natl Acad. Sci. USA 110, 12048–12053 (2013).
Bayer, A. et al. Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe 19, 705–712 (2016).
Zeldovich, V. B. et al. Placental syncytium forms a biophysical barrier against pathogen invasion. PLoS Pathog. 9, e1003821 (2013).
Tabata, T. et al. Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe http://dx.doi.org/10.1016/j.chom.2016.07.002 (2016).
Driggers, R. W. et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N. Engl. J. Med. 374, 2142–2151 (2016).
Miner, J. J. et al. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 165, 1081–1091 (2016).
Maltepe, E., Bakardjiev, A. I. & Fisher, S. J. The placenta: transcriptional, epigenetic, and physiological integration during development. J. Clin. Invest. 120, 1016–1025 (2010).
Kim, K. & Shresta, S. Neuroteratogenic viruses and lessons for Zika virus models. Trends Microbiol. 24, 622–636 (2016).
Quicke, K. M. et al. Zika virus infects human placental macrophages. Cell Host Microbe 20, 83–90 (2016).
Adams, K. M. & Nelson, J. L. Microchimerism: an investigative frontier in autoimmunity and transplantation. JAMA 291, 1127–1131 (2004).
Maidji, E., McDonagh, S., Genbacev, O., Tabata, T. & Pereira, L. Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis. Am. J. Pathol. 168, 1210–1226 (2006).
Guzman, M. G., Alvarez, M. & Halstead, S. B. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch. Virol. 158, 1445–1459 (2013).
de Alwis, R. et al. Dengue viruses are enhanced by distinct populations of serotype cross-reactive antibodies in human immune sera. PLoS Pathog. 10, e1004386 (2014).
Castanha, P. M. et al. Placental transfer of dengue virus (DENV)-specific antibodies and kinetics of DENV infection-enhancing activity in Brazilian infants. J. Infect. Dis. 214, 265–272 (2016).
Lanciotti, R. S. et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 14, 1232–1239 (2008).
Dejnirattisai, W. et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with Zika virus. Nat. Immunol. http://dx.doi.org/10.1038/ni.3515 (2016).
Priyamvada, L. et al. Human antibody responses after Dengue virus infection are highly cross-reactive to Zika virus. Proc. Natl Acad. Sci. USA 113, 7852–7857 (2016).
Swanstrom, J. A. et al. Dengue virus envelope dimer epitope monoclonal antibodies isolated from dengue patients are protective against Zika virus. mBio 7, e01123-16 (2016).
Stettler, K. et al. Specificity, cross-reactivity and function of antibodies elicited by Zika virus infection. Science http://dx.doi.org/10.1126/science.aaf8505 (2016).
Dancis, J., Lind, J., Oratz, M., Smolens, J. & Vara, P. Placental transfer of proteins in human gestation. Am. J. Obstet. Gynecol. 82, 167–171 (1961).
Bright, N. A. & Ockleford, C. D. Cytotrophoblast cells: a barrier to maternofetal transmission of passive immunity. J. Histochem. Cytochem. 43, 933–944 (1995).
Gusdon, J. P. Fetal and maternal immunoglobulin levels during pregnancy. Am. J. Obstet. Gynecol. 103, 895–900 (1969).
Garty, B. Z., Ludomirsky, A., Danon, Y. L., Peter, J. B. & Douglas, S. D. Placental transfer of immunoglobulin G subclasses. Clin. Diagn. Lab Immunol. 1, 667–669 (1994).
Malek, A., Sager, R., Kuhn, P., Nicolaides, K. H. & Schneider, H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am. J. Reprod. Immunol. 36, 248–255 (1996).
Dudley, D. M. et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat. Commun. 7, 12204 (2016).
Prisant, N. et al. Zika virus in the female genital tract. Lancet Infect. Dis. http://dx.doi.org/10.1016/S1473-3099(16)30193-1 (2016).
Mlakar, J. et al. Zika virus associated with microcephaly. N. Engl. J. Med. 374, 951–958 (2016).
Qian, X. et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238–1254 (2016).
Hanners, N. W. et al. Western Zika virus in human fetal neural progenitors persists long term with partial cytopathic and limited immunogenic effects. Cell Rep. 15, 2315–2322 (2016).
Tang, H. et al. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell 18, 587–590 (2016).
Garcez, P. P. et al. Zika virus impairs growth in human neurospheres and brain organoids. Science 352, 816–818 (2016).
Dang, J. et al. Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell http://dx.doi.org/10.1016/j.stem.2016.04.014 (2016).
Cugola, F. R. et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 534, 267–271 (2016).
Lazear, H. M. et al. A mouse model of Zika virus pathogenesis. Cell Host Microbe 19, 720–730 (2016).
Rossi, S. L. et al. Characterization of a novel murine model to study Zika virus. Am. J. Trop. Med. Hyg. 94, 1362–1369 (2016).
Aliota, M. T. et al. Characterization of lethal Zika virus infection in AG129 mice. PLoS Negl. Trop. Dis. 10, e0004682 (2016).
Dowall, S. D. et al. A susceptible mouse model for Zika virus infection. PLoS Negl. Trop. Dis. 10, e0004658 (2016).
Wu, K. Y. et al. Vertical transmission of Zika virus targeting the radial glial cells affects cortex development of offspring mice. Cell Res. 26, 645–654 (2016).
Schmidt, A., Morales-Prieto, D. M., Pastuschek, J., Frohlich, K. & Markert, U. R. Only humans have human placentas: molecular differences between mice and humans. J. Reprod. Immunol. 108, 65–71 (2015).
Wack, A., Terczynska-Dyla, E. & Hartmann, R. Guarding the frontiers: the biology of type III interferons. Nat. Immunol. 16, 802–809 (2015).
Li, C. et al. Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell 19, 120–126 (2016).
Dick, G. W. Zika virus. II. Pathogenicity and physical properties. Trans. R. Soc. Trop. Med. Hyg. 46, 521–534 (1952).
Paixao, E. S., Teixeira, M. G., Costa, M. D. & Rodrigues, L. C. Dengue during pregnancy and adverse fetal outcomes: a systematic review and meta-analysis. Lancet Infect. Dis. 16, 857–865 (2016).
Shepard, D. S., Undurraga, E. A., Halasa, Y. A. & Stanaway, J. D. The global economic burden of dengue: a systematic analysis. Lancet Infect. Dis. 16, 935–941 (2016).
Bhatt, S. et al. The global distribution and burden of dengue. Nature 496, 504–507 (2013).
Kinney, J. S. & Kumar, M. L. Should we expand the TORCH complex? A description of clinical and diagnostic aspects of selected old and new agents. Clin. Perinatol. 15, 727–744 (1988).
Musso, D. et al. Potential sexual transmission of Zika virus. Emerg. Infect. Dis. 21, 359–361 (2015).
Hills, S. L. et al. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission — continental United States, 2016. MMWR. Morb. Mortal. Wkly Rep. 65, 215–216 (2016).
Deckard, D. T. et al. Male-to-male sexual transmission of Zika virus — Texas, January 2016. MMWR. Morb. Mortal. Wkly Rep. 65, 372–374 (2016).
D'Ortenzio, E. et al. Evidence of sexual transmission of Zika virus. N. Engl. J. Med. 374, 2195–2198 (2016).
Foy, B. D. et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg. Infect. Dis. 17, 880–882 (2011).
Davidson, A., Slavinski, S., Komoto, K., Rakeman, J. & Weiss, D. Suspected female-to-male sexual transmission of Zika virus — New York City, 2016. MMWR. Morb. Mortal. Wkly Rep. 65, 716–717 (2016).
Mansuy, J. M. et al. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect. Dis. 16, 405 (2016).
Duffy, M. R. et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 360, 2536–2543 (2009).
Besnard, M. et al. Congenital cerebral malformations and dysfunction in fetuses and newborns following the 2013 to 2014 Zika virus epidemic in French Polynesia. Euro Surveill. http://dx.doi.org/10.2807/1560-7917.ES.2016.21.13.30181 (2016).
Liotta, G. et al. Elimination of mother-to-child transmission of HIV infection: the drug resource enhancement against AIDS and malnutrition model. Int. J. Environ. Res. Public Health 12, 13224–13239 (2015).
Stevens, J. & Lyall, H. Mother to child transmission of HIV: what works and how much is enough? J. Infect. 69 (Suppl. 1), S56–S62 (2014).
Perkins, T. A., Siraj, A. S., Ruktanonchai, C. W., Kraemer, M. U. G. & Tatem, A. J. Model-based projections of Zika virus infections in childbearing women in the Americas. Nat. Microbiol. 1, 16126 (2016).
Lambert, N., Strebel, P., Orenstein, W., Icenogle, J. & Poland, G. A. Rubella. Lancet 385, 2297–2307 (2015).
Manicklal, S., Emery, V. C., Lazzarotto, T., Boppana, S. B. & Gupta, R. K. The “silent” global burden of congenital cytomegalovirus. Clin. Microbiol. Rev. 26, 86–102 (2013).
Torgerson, P. R. & Mastroiacovo, P. The global burden of congenital toxoplasmosis: a systematic review. Bull. World Health Organ. 91, 501–508 (2013).
Rossant, J. & Cross, J. C. Placental development: lessons from mouse mutants. Nat. Rev. Genet. 2, 538–548 (2001).
Schleiss, M. R. Developing a vaccine against congenital cytomegalovirus (CMV) infection: what have we learned from animal models? where should we go next? Future Virol. 8, 1161–1182 (2013).
Carter, A. M. Animal models of human placentation — a review. Placenta 28 (Suppl. A), S41–S47 (2007).
World Health Organization. Zika situation report. WHO http://www.who.int/emergencies/zika-virus/situation-report/14-july-2016/en/ (2016).
Perez, S. et al. Confirmed case of Zika virus congenital infection, Spain, March 2016. Euro Surveill. http://dx.doi.org/10.2807/1560-7917.ES.2016.21.24.30261 (2016).
Culjat, M. et al. Clinical and imaging findings in an infant with Zika embryopathy. Clin. Infect. Dis. http://dx.doi.org/10.1093/cid/ciw324 (2016).
Aiken, A. R. et al. Requests for abortion in Latin America related to concern about Zika virus exposure. N. Engl. J. Med. 375, 396–398 (2016).
Oster, A. M. et al. Update: interim guidance for prevention of sexual transmission of Zika virus — United States, 2016. MMWR. Morb. Mortal. Wkly Rep. 65, 323–325 (2016).
Guttmacher Institute. Facts on abortion in Latin America and the Caribbean. Guttmacher Institute https://www.guttmacher.org/fact-sheet/facts-abortion-latin-america-and-caribbean (2015).
McConkey, C. A. et al. A three-dimensional culture system recapitulates placental syncytiotrophoblast development and microbial resistance. Sci. Adv. 2, e1501462 (2016).
Acknowledgements
The authors apologize to those whose work was not covered in detail owing to space restrictions and for inaccuracies or omissions resulting from the remarkably rapid pace of discovery in the Zika virus field. The authors thank Y. Sadovsky for helpful discussions in relation to this manuscript. C.B.C is supported by the US National Institutes of Health (NIH; grants R01-AI081759 and NIH R01-HD075665), and the Burroughs Wellcome Investigators in the Pathogenesis of Infectious Disease Award. H.M.L is supported by start-up funds from the University of North Carolina Department of Microbiology and Immunology and the Lineberger Comprehensive Cancer Center.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Coyne, C., Lazear, H. Zika virus — reigniting the TORCH. Nat Rev Microbiol 14, 707–715 (2016). https://doi.org/10.1038/nrmicro.2016.125
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro.2016.125
This article is cited by
-
Impact of chronic toxoplasmosis in pregnancy: association between maternal seropositivity for Toxoplasma gondii IgG antibodies and fetal growth restriction
Parasitology Research (2024)
-
Changes in ADAR RNA editing patterns in CMV and ZIKV congenital infections
BMC Genomics (2023)
-
Zika virus infection induces expression of NRF2 and antioxidant systems in trophoblast cells
Virus Genes (2023)
-
Zika virus-induced TNF-α signaling dysregulates expression of neurologic genes associated with psychiatric disorders
Journal of Neuroinflammation (2022)